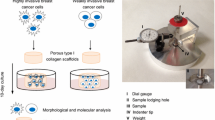
Abstract
Three-dimensional (3D) extracellular matrix (ECM) microenvironment is an important regulator of the stiffness of the tumors. Cancer cells require heterogeneous metabolic phenotypes to cope with resistance in the malignant process. However, how the stiffness of the matrix affects the metabolic phenotypes of cancer cells, is lacking. In this study, the young’s modulus of the synthesized collagen-chitosan scaffolds was adjusted according to the percentage ratio of collagen to chitosan. We cultured non-small cell lung cancer (NSCLC) cells in four different microenvironments (two-dimensional (2D) plates, stiffest 0.5–0.5 porous collagen-chitosan scaffolds, middle stiff 0.5–1 porous collagen-chitosan scaffolds, and softest 0.5–2 porous collagen-chitosan scaffolds) to investigate the influence of the difference of 2D and 3D cultures as well as the 3D scaffolds with different stiffnesses on the metabolic dependency of NSCLC cells. The results revealed that NSCLC cells cultured in 3D collagen-chitosan scaffolds displayed higher capacity of mitochondrial metabolism and fatty acid metabolism than that cultured in 2D culture. The metabolic response of NSCLC cells is differential for 3D scaffolds with different stiffnesses. The cells cultured in middle stiff 0.5–1 scaffolds displayed a higher potential of mitochondrial metabolism than that of stiffer 0.5–0.5 scaffolds and softer 0.5–2 scaffolds. Furthermore, NSCLC cells culture in 3D scaffolds displayed drug resistance compared with that in 2D culture which maybe via the hyperactivation of the mTOR pathway. Moreover, the cells cultured in 0.5–1 scaffolds showed higher ROS levels, which were counterbalanced by an equally high expression of antioxidant enzymes when compared to the cells grown in 2D culture, which may be regulated by the increased expression of PGC-1α. Together, these results demonstrate that differences in the microenvironments of cancer cells profoundly impact their metabolic dependencies.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Beloueche-Babari M, Casals Galobart T, Delgado-Goni T et al (2020) Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br J Cancer 122:895–903
Boroughs LK, DeBerardinis RJ (2015) Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 17:351–359
Chen X, Zhou L, Xu H et al (2020) Effect of the Application of a Dehydrothermal Treatment on the Structure and the Mechanical Properties of Collagen Film. Materials (Basel) 13:377
Chen W-L, Jin X, Wang M et al (2020) GLUT5-mediated fructose utilization drives lung cancer growth by stimulating fatty acid synthesis and AMPK/mTORC1 signaling. JCI Insight 5:e131596
Cox TR (2021) The matrix in cancer. Nat Rev Cancer 21:217–238
Cox TR, Erler JT (2011) Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 4:165–178
Cukierman E, Bassi DE (2010) Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol 20(3):139–45. https://doi.org/10.1016/j.semcancer.2010.04.004
Cunningham JT, Rodgers JT, Arlow DH et al (2007) mTOR controls mitochondrial oxidative function through a YY1–PGC-1α transcriptional complex. Nature 450:736–740
Currie E, Schulze A, Zechner R et al (2013) Cellular fatty acid metabolism and cancer. Cell Metab 18:153–161
Davidson SM, Papagiannakopoulos T, Olenchock BA et al (2016) Environment impacts the metabolic dependencies of Ras-driven non-small cell lung cancer. Cell Metab 23:517–528
Faubert B, Solmonson A, DeBerardinis RJ (2020) Metabolic reprogramming and cancer progression. Science (80- ) 368:eaaw5473
Gao Y, Peng K, Mitragotri S (2021) Covalently Crosslinked Hydrogels via Step-Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Adv Mater 33:2006362
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12:931–947
Gu L, Mooney DJ (2016) Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat Rev Cancer 16:56–66
Guri Y, Hall MN (2016) mTOR signaling confers resistance to targeted cancer drugs. Trends Cancer 2:688–697
Hayashida T, Wu M-H, Pierce A et al (2007) MAP-kinase activity necessary for TGF$β$1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J Cell Sci 120:4230–4240
He Q, Yang C, Xiang Z et al (2022) LINC00924-induced fatty acid metabolic reprogramming facilitates gastric cancer peritoneal metastasis via hnRNPC-regulated alternative splicing of Mnk2. Cell Death Dis 13:987
Honeder S, Tomin T, Nebel L et al (2021) Adipose Triglyceride lipase loss promotes a metabolic switch in A549 non–small cell lung cancer cell spheroids. Mol Cell Proteomics 20:100095
Janmey PA, Miller RT (2011) Mechanisms of mechanical signaling in development and disease. J Cell Sci 124:9–18
Kapałczyńska M, Kolenda T, Przybyła W et al (2018) 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch Med Sci 14:910
Kim I-Y, Seo S-J, Moon H-S et al (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26:1–21
Li X, Wang J (2020) Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis. Int J Biol Sci 16:2014–2028
Li J, Huang Q, Long X et al (2015) CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J Hepatol 63:1378–1389
Lu T, Sun L, Wang Z et al (2019) Fatty acid synthase enhances colorectal cancer cell proliferation and metastasis via regulating AMPK/mTOR pathway. Onco Targets Ther 12:3339–3347
Madak-Erdogan Z, Band S, Zhao YC et al (2019) Free Fatty Acids Rewire Cancer Metabolism in Obesity-Associated Breast Cancer via Estrogen Receptor and mTOR Signaling. Cancer Res 79:2494–2510
Martinez-Pacheco S, O’Driscoll L (2021) Pre-Clinical In Vitro Models Used in Cancer Research: Results of a Worldwide Survey. Cancers (Basel) 13:6033
Mohammadi H, Sahai E (2018) Mechanisms and impact of altered tumour mechanics. Nat Cell Biol 20:766–774
Nguyen-Ngoc K-V, Cheung KJ, Brenot A et al (2012) ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci 109:E2595–E2604
Ozel D, Bayraktarli RY, Coskun ZU Re: Shear wave elastography for localization of prostate cancer lesions and assessment of elasticity thresholds: implications for targeted biopsies and active surveillance protocols. J Urol 193:7947–800
Padanad MS, Konstantinidou G, Venkateswaran N et al (2016) Fatty acid oxidation mediated by Acyl-CoA synthetase long chain 3 is required for mutant KRAS lung tumorigenesis. Cell Rep 16:1614–1628
Park JS, Burckhardt CJ, Lazcano R et al (2020) Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 578:621–626
Plodinec M, Loparic M, Monnier CA et al (2012) The nanomechanical signature of breast cancer. Nat Nanotechnol 7:757–765
Raftery RM, Woods B, Marques ALP et al (2016) Multifunctional biomaterials from the sea: Assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality. Acta Biomater 43:160–169
Ramanathan A, Schreiber SL (2009) Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci 106:22229–22232
Salvi AM, DeMali KA (2018) Mechanisms linking mechanotransduction and cell metabolism. Curr Opin Cell Biol 54:114–120
Samani A, Zubovits J, Plewes D (2007) Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol 52:1565–1576
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462
Soo JY-C, Jansen J, Masereeuw R, Little MH (2018) Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat Rev Nephrol 14:378–393
St-Pierre J, Drori S, Uldry M et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408
Szatrowski TP, Nathan CF (1991) Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51:794–798
Tan W, Krishnaraj R, Desai TA (2001) Evaluation of nanostructured composite collagen–chitosan matrices for tissue engineering. Tissue Eng 7:203–210
Tanner LB, Goglia AG, Wei MH et al (2018) Four key steps control glycolytic flux in mammalian cells. Cell Syst 7:49–62
Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK (2016) Extracellular matrix structure. Adv Drug Deliv Rev 97:4–27
Ulrich TA, de Juan Pardo EM, Kumar S (2009) The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 69:4167–4174
Vazquez F, Lim J-H, Chim H et al (2013) PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 23:287–301
Xu K, Xia P, Chen X et al (2023) ncRNA-mediated fatty acid metabolism reprogramming in HCC. Trends Endocrinol Metab 6:S1043-2760
Yang Y, Ishak Gabra MB, Hanse EA et al (2019) MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun 10:1–15
Yu H, Mouw JK, Weaver VM (2011) Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol 21:47–56
Wang F, Wang M, She Z et al (2015) Collagen/chitosan based two-compartment and bi-functional dermal scaffolds for skin regeneration. Mater Sci Eng C 52:155–162
Wellman P, Howe RD, Dalton E, Kern KA (1999) Breast tissue stiffness in compression is correlated to histological diagnosis. Harvard BioRobotics Lab Tech Rep 1
Acknowledgements
Xiaorong Fu acknowledges support from the China Scholarship Council (grant number 201906050131).
Funding
This work was supported by the Japan Society for the Promotion of Science under the Grants-in-Aid for Scientific Research (S) (No. 17H06146).
Author information
Authors and Affiliations
Contributions
X. F.: Investigation, Methodology, Visualization, Writing-original draft, Writing- review, and editing. Y. K.: Resources, Investigation, Methodology, Writing–Review, and Editing. Y. T.: Resources, writing–review, and editing. G. S.: Resources, writing review, and editing. Y. J.: Conceptualization, supervision, writing–original draft, writing–review & editing, funding acquisition. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• The microenvironment of 3D collagen-chitosan scaffolds reprograms the metabolic requirement of NSCLC cells.
• NSCLC cells cultured in 3D collagen-chitosan scaffolds revealed higher capacity of mitochondrial metabolism and fatty acid metabolism than that cultured in 2D plate.
• Metabolic dependency of NSCLC cells responded to the change of stiffness of 3D scaffolds
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, X., Kimura, Y., Toku, Y. et al. Metabolic dependency of non-small cell lung cancer cells affected by three-dimensional scaffold and its stiffness. J Physiol Biochem 79, 597–611 (2023). https://doi.org/10.1007/s13105-023-00960-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00960-6