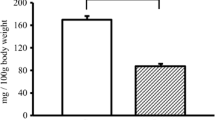
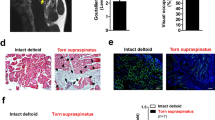
Abstract
Aquaporins (AQPs) are water channels in the cell membrane that regulate osmosis in response to rapid changes in intracellular and extracellular fluid concentration caused by extrinsic factors. While there are so many studies on the association of AQPs with muscular atrophy, sarcopenia, and Duchenne muscular dystrophy (DMD), the expression of AQP has not been verified in naturally aging mice or humans. Notably, due to the characteristics of AQPs, the difference in function cannot be evaluated without extrinsic factors such as acute water restriction. The purpose of this study was to investigate the changes in AQPs expression and function due to natural aging under acute water restriction conditions in aging mice. The expression of AQP4 was shown to decrease with aging similar to previous studies. However, for the first time, this study results confirmed that AQP1 expression increased in aging mice. In addition, the expression of Aqp1 decreased in the acute water restricted group compared to the control group after acute water restriction in aging mice. These results suggest that although the expression of AQP1 increases with aging, its function is reduced. We also confirmed that overexpression of Aqp1 can inhibit myotube differentiation and that knockdown can promote myotube differentiation through in vitro experiments. In conclusion, based on our results, we suggest that the AQP1 is an important factor in sarcopenia caused by natural aging accompanied by chronic dehydration.







Similar content being viewed by others
References
Agre P (2006) The aquaporin water channels. Proc Am Thorac Soc 3:5–13. https://doi.org/10.1513/pats.200510-109JH
Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S (2002) Aquaporin water channels--from atomic structure to clinical medicine. J Physiol 542:3–16. https://doi.org/10.1113/jphysiol.2002.020818
Au CG, Butler TL, Egan JR, Cooper ST, Lo HP, Compton AG, North KN, Winlaw DS (2008) Changes in skeletal muscle expression of AQP1 and AQP4 in dystrophinopathy and dysferlinopathy patients. Acta Neuropathol 116:235–246. https://doi.org/10.1007/s00401-008-0369-z
Au CG, Cooper ST, Lo HP, Compton AG, Yang N, Wintour EM, North KN, Winlaw DS (2004) Expression of aquaporin 1 in human cardiac and skeletal muscle. J Mol Cell Cardiol 36:655–662. https://doi.org/10.1016/j.yjmcc.2004.01.009
Baek KW, Jung YK, Kim JS, Park JS, Hah YS, Kim SJ, Yoo JI (2020) Rodent model of muscular atrophy for sarcopenia study. J Bone Metab 27:97–110. https://doi.org/10.11005/jbm.2020.27.2.97
Baek KW, Jung YK, Park JS, Kim JS, Hah YS, Kim SJ, Yoo JI (2021) Two types of mouse models for sarcopenia research: senescence acceleration and genetic modification models. J Bone Metab 28:179–191. https://doi.org/10.11005/jbm.2021.28.3.179
Baek KW, Kim JS, Park JS, Kim SJ, Ha YC, Jeong OY, Yoo JI (2020) Validation of dual energy X-ray absorptiometry and nuclear magnetic resonance in the analysis of body composition in mice. J Bone Metab 27:291–299. https://doi.org/10.11005/jbm.2020.27.4.291
Baek KW, Lee DI, Jeong MJ, Kang SA, Choe Y, Yoo JI, Yu HS, Kim JS (2020) Effects of lifelong spontaneous exercise on the M1/M2 macrophage polarization ratio and gene expression in adipose tissue of super-aged mice. Exp Gerontol 141:111091. https://doi.org/10.1016/j.exger.2020.111091
Bekkevold CM, Robertson KL, Reinhard MK, Battles AH, Rowland NE (2013) Dehydration parameters and standards for laboratory mice. J Am Assoc Lab Anim Sci 52:233–239
Crosbie RH, Dovico SA, Flanagan JD, Chamberlain JS, Ownby CL, Campbell KP (2002) Characterization of aquaporin-4 in muscle and muscular dystrophy. FASEB J 16:943–949. https://doi.org/10.1096/fj.01-0327com
Dmitrieva NI, Burg MB (2011) Increased insensible water loss contributes to aging related dehydration. PLoS One 6:e20691. https://doi.org/10.1371/journal.pone.0020691
Frigeri A, Nicchia GP, Balena R, Nico B, Svelto M (2004) Aquaporins in skeletal muscle: reassessment of the functional role of aquaporin-4. FASEB J 18:905–907. https://doi.org/10.1096/fj.03-0987fje
Frigeri A, Nicchia GP, Nico B, Quondamatteo F, Herken R, Roncali L, Svelto M (2001) Aquaporin-4 deficiency in skeletal muscle and brain of dystrophic mdx mice. FASEB J 15:90–98. https://doi.org/10.1096/fj.00-0260com
Frigeri A, Nicchia GP, Verbavatz JM, Valenti G, Svelto M (1998) Expression of aquaporin-4 in fast-twitch fibers of mammalian skeletal muscle. J Clin Invest 102:695–703. https://doi.org/10.1172/JCI2545
Hamalainen N, Pette D (1993) The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem 41:733–743. https://doi.org/10.1177/41.5.8468455
Hosaka Y, Yokota T, Miyagoe-Suzuki Y, Yuasa K, Imamura M, Matsuda R, Ikemoto T, Kameya S, Takeda S (2002) Alpha1-syntrophin-deficient skeletal muscle exhibits hypertrophy and aberrant formation of neuromuscular junctions during regeneration. J Cell Biol 158:1097–1107. https://doi.org/10.1083/jcb.200204076
Hua Y, Ying X, Qian Y, Liu H, Lan Y, Xie A, Zhu X (2019) Physiological and pathological impact of AQP1 knockout in mice. Biosci Rep 39. https://doi.org/10.1042/BSR20182303
Ishido M, Nakamura T (2017) Marked decrease of aquaporin-4 protein is independent of the changes in alpha1-syntrophin and TRPV4 levels in response to denervation-induced muscle atrophy in vivo. J Muscle Res Cell Motil 38:175–181. https://doi.org/10.1007/s10974-017-9471-y
Ishido M, Nakamura T (2020) Time course changes in AQP4 expression patterns in progressive skeletal muscle atrophy during the early stage of denervation. J Musculoskelet Neuronal Interact 20:114–120
Kanasi E, Ayilavarapu S, Jones J (2016) The aging population: demographics and the biology of aging. Periodontol 72:13–18. https://doi.org/10.1111/prd.12126
Kingsley J, Torimoto K, Hashimoto T, Eguchi S (2021) Angiotensin II inhibition: a potential treatment to slow the progression of sarcopenia. Clin Sci (Lond) 135:2503–2520. https://doi.org/10.1042/CS20210719
Lloyd N (2016) AIM coalition announces establishment of ICD-10-CM code for sarcopenia by the centers for disease control and prevention. https://www.prweb.com/releases/2016/04/prweb13376057.htm. Accessed Nov 3 2021
Magni F, Chinello C, Raimondo F, Mocarelli P, Kienle MG, Pitto M (2008) AQP1 expression analysis in human diseases: implications for proteomic characterization. Expert Rev Proteomics 5:29–43. https://doi.org/10.1586/14789450.5.1.29
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S–991S. https://doi.org/10.1093/jn/127.5.990S
Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP (1985) Effect of exercise on longevity, body weight, locomotor performance, and passive-avoidance memory of C57BL/6J mice. Neurobiol Aging 6:17–24. https://doi.org/10.1016/0197-4580(85)90066-1
Santilli V, Bernetti A, Mangone M, Paoloni M (2014) Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 11:177–180
Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R (2017) Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord 16:21. https://doi.org/10.1186/s40200-017-0302-x
Silver AJ, Flood JF, Morley JE (1991) Effect of aging on fluid ingestion in mice. J Gerontol 46:B117–B121. https://doi.org/10.1093/geronj/46.3.b117
Wakayama Y, Hirako S, Ogawa T, Jimi T, Shioda S (2014) Upregulated expression of AQP 7 in the skeletal muscles of obese ob/ob mice. Acta Histochem Cytochem 47:27–33. https://doi.org/10.1267/ahc.13032
Wakayama Y, Inoue M, Kojima H, Jimi T, Shibuya S, Hara H, Oniki H (2004) Expression and localization of aquaporin 7 in normal skeletal myofiber. Cell Tissue Res 316:123–129. https://doi.org/10.1007/s00441-004-0857-y
Wakayama Y, Jimi T, Inoue M, Kojima H, Murahashi M, Kumagai T, Yamashita S, Hara H, Shibuya S (2002) Reduced aquaporin 4 expression in the muscle plasma membrane of patients with Duchenne muscular dystrophy. Arch Neurol 59:431–437. https://doi.org/10.1001/archneur.59.3.431
Yoo JI, Choi H, Song SY, Park KS, Lee DH, Ha YC (2018) Relationship between water intake and skeletal muscle mass in elderly Koreans: a nationwide population-based study. Nutrition 53:38–42. https://doi.org/10.1016/j.nut.2018.01.010
Yoo JI, Kim MJ, Na JB, Chun YH, Park YJ, Park Y, Hah YS, Ha YC, Park KS (2018) Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J Cachexia Sarcopenia Muscle 9:1034–1041. https://doi.org/10.1002/jcsm.12340
Zadrozniak A, Wojda E, Wlaz A, Luszczki JJ (2009) Characterization of acute adverse-effect profiles of selected antiepileptic drugs in the grip-strength test in mice. Pharmacol Rep 61:737–742. https://doi.org/10.1016/s1734-1140(09)70128-8
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1F1A1059231). This study was conducted with support from the Rural Development Administration (PJ014155052019).
Author information
Authors and Affiliations
Contributions
SJK and KWB: conception and design, animal experiment, data collection, figure preparation, and manuscript writing. YKJ, JSK, and BGK: animal experiment, in vitro experiment, and histological experiment. HSY, JSP, and JIY: conception and financial support. JIY: conception and design, data interpretation, and final approval of the manuscript. All the authors have reviewed and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The animal studies were approved by the Institutional Animal Care and Use and Committee of Pusan National University (approval number, PNU-2019-2358).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• By natural aging, the expression of AQP1 increases, AQP4 decreases, and AQP7 does not appear to change.
• In aged mice, expression of AQP1 significantly decreased gene expression due to acute water restriction, but protein expression was not.
• AQP1 overexpression inhibits myotube differentiation and knockdown promotes myotube differentiation.
Supplementary information

Fig. S1
AQPs expression on the IHC microscopic images of GA muscle sections. Each arrow indicate AQP1 stained location. Scale bar represents 60 μm (PNG 2293 kb)

Fig. S2
AQPs expression on the IHC microscopic images of TA muscle sections. The arrows indicate AQP1 stained locations. Scale bar represents 60 μm (PNG 2237 kb)

Fig. S3
Transfection efficiency of Aqp1. (A) Aqp1 overexpression and (B) Aqp1 knockdown. Transfection efficiency was analyzed by quantitative real-time RT-PCR. All data are presented as the mean ± S.D.; vs. control group, **p < 0.01, ****p < 0.0001 (PNG 31 kb)

Fig. S4
Myotube differentiation according to passage of the day after transfection. Each arrow indicates differentiated myotube. Scale bar represents 1150 μm (PNG 6066 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, SJ., Baek, KW., Jung, YK. et al. Changes in aquaporins expression due to acute water restriction in naturally aging mice. J Physiol Biochem 79, 71–81 (2023). https://doi.org/10.1007/s13105-022-00921-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00921-5