Abstract
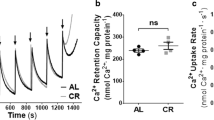
The antioxidant role of mitochondrial uncoupling protein 3 (UCP3) is controversial. This work aimed to investigate the effects of UCP3 on the heart of mice housed at thermoneutral temperature, an experimental condition that avoids the effects of thermoregulation on mitochondrial activity and redox homeostasis, preventing the alterations related to these processes from confusing the results caused by the lack of UCP3. WT and KO UCP3 mice were acclimatized at 30 °C for 4 weeks and hearts were used to evaluate metabolic capacity and redox state. Tissue and mitochondrial respiration, the activities of the mitochondrial complexes, and the protein expression of mitochondrial complexes markers furnished information on mitochondrial functionality. The levels of lipid and protein oxidative damage markers, the activity of antioxidant enzymes, the reactive oxygen species levels, and the susceptibility to in vitro Fe-ascorbate-induced oxidative stress furnished information on redox state. UCP3 ablation reduced tissue and mitochondrial respiratory capacities, not affecting the mitochondrial content. In KO UCP3 mice, the mitochondrial complexes activities were lower than in WT without changes in their content. These effects were accompanied by an increase in the level of oxidative stress markers, ROS content, and in vitro susceptibility to oxidative stress, notwithstanding that the activities of antioxidant enzymes were not affected by UCP3 ablation. Such modifications are also associated with enhanced activation/phosphorylation of EIF2α, a marker of integrated stress response and endoplasmic reticulum stress (GRP778 BIP). The lack of UCP3 makes the heart more prone to oxidative insult by reducing oxygen consumption and increasing ROS. Our results demonstrate that UCP3 helps the cell to preserve mitochondrial function by mitigating oxidative stress.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Code availability
Not applicable.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Barré H, Bailly L, Rouanet JL (1987) Increased oxidative capacity in skeletal muscles from acclimated ducklings: a comparison with rats. Comp Biochem Physiol 88B:519–522. https://doi.org/10.1016/0305-0491(87)90337-3
Brand MD, Pamplona R, Portero-Otín M, Requena JR, Roebuck SJ, Buckingham JA, Clapham JC, Cadenas S (2002) Oxidative damage and phospholipid fatty acyl composition in skeletal muscle mitochondria from mice underexpressing or overexpressing uncoupling protein 3. Biochem J 368: 597–603. https://doi.org/10.1042/BJ20021077
Cadenas S (2018) Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg 1859:940–950. https://doi.org/10.1016/j.bbabio.2018.05.019
Cadenas S, Echtay KS, Harper JA, Jekabsons MB, Buckingham JA, Grau E, Abuin A, Chapman H, Clapham JC, Brand MD (2002) The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J Biol Chem 277:2773–7778. https://doi.org/10.1074/jbc.M109736200
Cannon B, Nedergaard J (2011) Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214:242–253. https://doi.org/10.1242/jeb.050989
Di Meo S, Venditti P, De Leo T (1996) Tissue protection against oxidative stress. Experientia 52:786–794. https://doi.org/10.1007/BF01923990
Dilberger B, Baumanns S, Schmitt F, Schmiedl T, Hardt M, Wenzel U, Eckert GP (2019) Mitochondrial oxidative stress impairs energy metabolism and reduces stress resistance and longevity of C. elegans. Oxid Med Cell Longev. https://doi.org/10.1155/2019/6840540
Driver AS, Kodavanti PRS, Mundy WR (2000) Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 22:175–181. https://doi.org/10.1016/s0892-0362(99)00069-0
Dudele A, Rasmussen GM, Mayntz D, Malte H, Lund S, Wang T (2015) Effects of ambient temperature on glucose tolerance and insulin sensitivity test outcomes in normal and obese C57 male mice. Physiol Rep. https://doi.org/10.14814/phy2.12396
Fan F, Duan Y, Yang F, Trexler C, Wang H, Huang L, Li Y, Tang H, Wang G, Fang X, Liu J, Jia N, Chen J (2020) Ouyang K. Deletion of heat shock protein 60 in adult mouse cardiomyocytes perturbs mitochondrial protein homeostasis and causes heart failure. Cell Death Differ 27:587–600
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9:203–209. https://doi.org/10.1016/j.cmet.2008.12.014
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–120. https://doi.org/10.1016/s0076-6879(84)05015-1
Flohé L (1984) Otting F Superoxide dismutase assays. Methods Enzymol 105:93–104. https://doi.org/10.1016/s0076-6879(84)05013-8
Flögel U, Gödecke A, Klotz LO, Schrader J (2004) Role of myoglobin in the antioxidant defense of the heart. FASEB J 18:1156–1158. https://doi.org/10.1096/fj.03-1382fje
Forsström S, Jackson CB, Carroll CJ, Kuronen M, Pirinen E, Pradhan S, Marmyleva A, Auranen M, Kleine IM, Khan NA, Roivainen A, Marjamäki P, Liljenbäck H, Wang L, Battersby BJ, Richter U, Velagapudi V, Nikkanen J, Euro L, Suomalainen A (2019) Fibroblast growth factor 21 drives dynamics of local and systemic stress responses in mitochondrial myopathy with mtDNA deletions. Cell Metab 30:1040–1054
Goglia F, Skulachev VP (2003) A function for novel uncoupling proteins: antioxidant defense of mitochondrial matrix by translocating fatty acid peroxides from the inner to the outer membrane leaflet. FASEB J 17:1585–1591. https://doi.org/10.1096/fj.03-0159hyp
Gong DW, Monemdjou S, Gavrilova O, Leon LR, Marcus-Samuels B, Chou CJ, Everett C, Kozak LP, Li C, Deng C, Harper ME, Reitman ML (2000) Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J Biol Chem 275:16251–16257. https://doi.org/10.1074/jbc.M910177199
Heath RL, Tappel AL (1976) A new sensitive assay for the measurement of hydroperoxides. Anal Biochem 76:184–191. https://doi.org/10.1016/0003-2697(76)90277-3
Klingenberg M, Slenczka W (1959) Pyridine nucleotide in liver mitochondria. An analysis of their redox relationships. Biochem Z 331:486–517
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18. https://doi.org/10.1016/s0014-5793(97)01159-9
Krauss S, Zhang CY, Lowell BB (2005) The mitochondrial uncoupling protein homologues. Nat Rev Mol Cell Biol 6:248–261. https://doi.org/10.1038/nrm1592
Laszczyca P, Kawka-Serweciňska E, Witas I, Doležych B, Migula P (1995) Iron ascorbate-stimulated lipid peroxidation in vitro. Why is the method controversial? Gen Physiol Biophys 14:3–18
Lombardi A, Busiello RA, Napolitano L, Cioffi F, Moreno M, de Lange P, Silvestri E, Lanni A, Goglia F (2010) UCP3 translocates lipid hydroperoxide and mediates lipid hydroperoxide-dependent mitochondrial uncoupling. J Biol Chem 285:16599–16605. https://doi.org/10.1074/jbc.M110.102699
Melber A, Haynes C (2018) UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res 28:281–295
Musatov A, Robinson NC (2012) Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radical Re 46:1313–1326. https://doi.org/10.3109/10715762.2012.717273
Nabben M, Shabalina IG, Moonen-Kornips E, van Beurden D, Cannon B, Schrauwen P, Nedergaard J, Hoeks J (2011) Uncoupled respiration, ROS production, acute lipotoxicity and oxidative damage in isolated skeletal muscle mitochondria from UCP3-ablated mice. Biochim Biophys Acta 1807. https://doi.org/10.1016/j.bbabio.2011.04.003
Ozcan C, Palmeri M, Horvath TL, Russell KS, Russell RR 3rd (2013) Role of uncoupling protein 3 in ischemia-reperfusion injury, arrhythmias, and preconditioning. Am J Physiol Heart Circ Physiol 304: H1192–200. https://doi.org/10.1152/ajpheart.00592.2012
Palmieri F (2013) The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med 34:465–484. https://doi.org/10.1016/j.mam.2012.05.005
Perrino C, Schiattarella GG, Sannino A, Pironti G, Petretta MP, Cannavo A, Gargiulo G, Ilardi F, Magliulo F, Franzone A, Carotenuto G, Serino F, Altobelli GG, Cimini V, Cuocolo A, Lombardi A, Goglia F, Indolfi C, Trimarco B, Esposito G (2013) Genetic deletion of uncoupling protein 3 exaggerates apoptotic cell death in the ischemic heart leading to heart failure. J Am Heart Assoc. https://doi.org/10.1161/JAHA.113.000086
Ragan MT, Ragan VM, Wilson PN, Darley-Usmar PN (1987) Lower sub-fractionation of mitochondria and isolation of the proteins of oxidative phosphorylation in Darley-Usmar VMD, Rickwood D, Wilson MT, editors. Mitochondria: a practical approach, Oxford: IRL Pres. p 79–112
Ricquier D (2017) UCP1, the mitochondrial uncoupling protein of brown adipocyte: a personal contribution and a historical perspective. Biochimie 134:3–8. https://doi.org/10.1016/j.biochi.2016.10.018
Rolfe DF, Hulbert AJ, Brand MD (1994) Characteristics of mitochondrial proton leak and control of oxidative phosphorylation in the major oxygen-consuming tissues of the rat. Biochim Biophys Acta 1188:405–416. https://doi.org/10.1016/0005-2728(94)90062-0
Schild L, Reinheckel T, Wiswedel I, Augustin W (1997) Short-term impairment of energy production in isolated rat liver mitochondria by hypoxia/reoxygenation: involvement of oxidative protein modification. Biochem J 328:205–210. https://doi.org/10.1042/bj3280205
Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM (2008) Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 294:H1581–H1588. https://doi.org/10.1152/ajpheart.01000.2007
Toime LJ, Brand MD (2000) Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radical Biol Med 49:606–611. https://doi.org/10.1016/j.freeradbiomed.2010.05.010
Venditti P, Napolitano G, Di Stefano L, Agnisola C, Di Meo S (2011) Effect of vitamin E administration on response to ischaemia-reperfusion of hearts from cold-exposed rats. Exp Physiol 96:635–46. https://doi.org/10.1113/expphysiol.2011.058289
Venditti P, Napolitano G, Fasciolo G, Di Meo S (2019) Thyroid state affects H2O2 removal by rat heart mitochondria. Arch Biochem Biophys 662:61–67. https://doi.org/10.1016/j.abb.2018.11.025
Vögtle FN (2021) Open questions on the mitochondrial unfolded protein response. FEBS J 288:2856–2869
Zhang Z, Zhang L, Zhou L, Lei Y, Zhang Y, Huang C (2019) Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol 25:101047. https://doi.org/10.1016/j.redox.2018.11.005
Funding
This work was supported by grants from the University of Naples Federico II [Ricerca Dipartimentale 2018–2020].
Author information
Authors and Affiliations
Contributions
N.G., L.A., G.F., and V.P. conceived and designed the experiments; F.G. and M.N. carried out all the animal studies and performed the experiments; G.N., G.F., L.A., and V.P. analyzed the data; G.F., L.A., and V.P. wrote the manuscript; all the authors revised the manuscript.
The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal protocols were approved by the Committee on the Ethics of Animal Experiments of the University of Naples Federico II and the Italian Minister of Health. Every effort was made to minimize animal pain and suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• UCP3 ablation increases ROS content and reduces mitochondrial respiration
• KO UCP3 mice mitochondria show a high susceptibility to oxidative stress
• UCP3 regulates the ROS level in the heart of mice kept at thermoneutrality
Rights and permissions
About this article
Cite this article
Napolitano, G., Fasciolo, G., Magnacca, N. et al. Oxidative damage and mitochondrial functionality in hearts from KO UCP3 mice housed at thermoneutrality. J Physiol Biochem 78, 415–425 (2022). https://doi.org/10.1007/s13105-022-00882-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00882-9