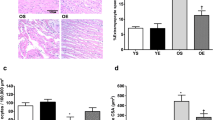
Abstract
Aerobic exercise training induces a unique cardioprotective phenotype, but it is becoming clear that it does not promote the same structural, functional, and molecular adaptations in both ventricles. In the present study, we aimed to better characterize and compare the molecular pathways involved in the exercise-induced remodeling of both ventricles. Female Sprague-Dawley rats were randomly assigned to control and exercise groups. Animals in the exercise group were submitted to low-intensity treadmill exercise for 54 weeks. After the experimental period, biventricular hemodynamic analysis was performed and right and left ventricles were harvested for morphological and biochemical analyses. Data showed that long-term low-intensity exercise training improves cardiac function, especially left ventricular diastolic function; however, the expression of connexin-43, CCAAT-enhancer binding protein β, and c-kit did not change in none of the ventricles. In the right ventricle, long-term exercise training induced an increase of manganese superoxide dismutase and sirtuin 3 protein expression, suggestive of improved antioxidant capacity. Our results also support that long-term aerobic exercise training imposes greater metabolic remodeling to the right ventricle, mainly by increasing mitochondrial ability to produce ATP, with no association to estrogen-related receptor α regulation.




Similar content being viewed by others
References
Aaron CP, Tandri H, Barr RG, Johnson WC, Bagiella E, Chahal H, Jain A, Kizer JR, Bertoni AG, Lima JAC, Bluemke DA, Kawut SM (2011) Physical activity and right ventricular structure and function: the MESA-Right Ventricle Study. Am J Respir Crit Care Med 183:396–404. https://doi.org/10.1164/rccm.201003-0469OC
Amado FM, Barros A, Azevedo AL, Vitorino R, Ferreira R (2014) An integrated perspective and functional impact of the mitochondrial acetylome. Expert Rev Proteomics 11:383–394. https://doi.org/10.1586/14789450.2014.899470
Antunes D, Padrao AI, Maciel E, Santinha D, Oliveira P, Vitorino R, Moreira-Goncalves D, Colaco B, Pires MJ, Nunes C, Santos LL, Amado F, Duarte JA, Domingues MR, Ferreira R (2014) Molecular insights into mitochondrial dysfunction in cancer-related muscle wasting. Biochim Biophys Acta 1841:896–905. https://doi.org/10.1016/j.bbalip.2014.03.004
Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ, Levine BD (2014) Cardiac remodeling in response to 1 year of intensive endurance training. Circulation 130:2152–2161. https://doi.org/10.1161/CIRCULATIONAHA.114.010775
Bal MP, de Vries WB, van der Leij FR, van Oosterhout MF, Baan J, van der Wall EE, van Bel F, Steendijk P (2005) Left ventricular pressure-volume relationships during normal growth and development in the adult rat-studies in 8- and 50-week-old male Wistar rats. Acta Physiol Scand 185:181–191. https://doi.org/10.1111/j.1365-201X.2005.01484.x
Beery AK, Zucker I (2011) Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35:565–572. https://doi.org/10.1016/j.neubiorev.2010.07.002
Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S, Mont L (2011) Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 123:13–22. https://doi.org/10.1161/CIRCULATIONAHA.110.938282
Bishop DJ, Granata C, Eynon N (2014) Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim Biophys Acta 1840:1266–1275. https://doi.org/10.1016/j.bbagen.2013.10.012
Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM (2010) C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143:1072–1083. https://doi.org/10.1016/j.cell.2010.11.036
Chang Y, Yu T, Yang H, Peng Z (2015) Exhaustive exercise-induced cardiac conduction system injury and changes of cTnT and Cx43. Int J Sports Med 36:1–8. https://doi.org/10.1055/s-0034-1384545
Clayton JA, Collins FS (2014) NIH to balance sex in cell and animal studies. Nature 509:282–283
Coore HG, Denton RM, Martin BR, Randle PJ (1971) Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J 125:115–127. https://doi.org/10.1042/bj1250115
Ferreira R, Vitorino R, Padrao AI, Espadas G, Mancuso FM, Moreira-Goncalves D, Castro-Sousa G, Henriques-Coelho T, Oliveira PA, Barros AS, Duarte JA, Sabido E, Amado F (2014) Lifelong exercise training modulates cardiac mitochondrial phosphoproteome in rats. J Proteome Res 13:2045–2055. https://doi.org/10.1021/pr4011926
Ferreira R, Moreira-Goncalves D, Azevedo AL, Duarte JA, Amado F, Vitorino R (2015) Unraveling the exercise-related proteome signature in heart. Basic Res Cardiol 110:454. https://doi.org/10.1007/s00395-014-0454-5
Ferreira R, Nogueira-Ferreira R, Trindade F, Vitorino R, Powers SK, Moreira-Gonçalves D (2018) Sugar or fat: the metabolic choice of the trained heart. Metabol Clin Exp 87:98–104. https://doi.org/10.1016/j.metabol.2018.07.004
Finocchiaro G, Dhutia H, D’Silva A, Malhotra A, Steriotis A, Millar L, Prakash K, Narain R, Papadakis M, Sharma R, Sharma S (2017) Effect of sex and sporting discipline on LV adaptation to exercise. JACC Cardiovasc Imaging 10:965–972. https://doi.org/10.1016/j.jcmg.2016.08.011
Foryst-Ludwig A, Kreissl MC, Sprang C, Thalke B, Böhm C, Benz V, Gürgen D, Dragun D, Schubert C, Mai K, Stawowy P, Spranger J, Regitz-Zagrosek V, Unger T, Kintscher U (2011) Sex differences in physiological cardiac hypertrophy are associated with exercise-mediated changes in energy substrate availability. Am J Physiol Heart Circ Physiol 301:H115–H122. https://doi.org/10.1152/ajpheart.01222.2010
Heidbuchel H, Hoogsteen J, Fagard R, Vanhees L, Ector H, Willems R, Van Lierde J (2003) High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J 24:1473–1480. https://doi.org/10.1016/S0195-668X(03)00282-3
Heiskanen MA, Leskinen T, Heinonen IHA, Löyttyniemi E, Eskelinen J-J, Virtanen K, Hannukainen JC, Kalliokoski KK (2016) Right ventricular metabolic adaptations to high-intensity interval and moderate-intensity continuous training in healthy middle-aged men. Am J Physiol Heart Circ Physiol 311:H667–H675. https://doi.org/10.1152/ajpheart.00399.2016
Jones SA (2006) Ageing to arrhythmias: conundrums of connections in the ageing heart. J Pharm Pharmacol 58:1571–1576. https://doi.org/10.1211/jpp.58.12.0002
Kararigas G, Leber J, Schubert C, Fliegner D, Mahmoodzadeh S, Regitz-Zagrosek V, Dworatzek E, Kusch A, Dragun D, Westphal C, Moulin M, Ventura-Clapier R, Gustafsson J-A, Davidson MM (2014) Sex differences in exercise-induced physiological myocardial hypertrophy are modulated by oestrogen receptor beta. Cardiovasc Res 102:418–428. https://doi.org/10.1093/cvr/cvu065
La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbuchel H, Prior DL (2012) Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 33:998–1006. https://doi.org/10.1093/eurheartj/ehr397
La Gerche A, Roberts T, Claessen G (2014) The response of the pulmonary circulation and right ventricle to exercise: exercise-induced right ventricular dysfunction and structural remodeling in endurance athletes (2013 Grover Conference series). Pulm Circ 4:407–416. https://doi.org/10.1086/677355
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM (2009) Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 119:2663–2670. https://doi.org/10.1161/CIRCULATIONAHA.108.838698
Lawler JM, Powers SK, Hammeren J, Martin AD (1993) Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med Sci Sports Exerc 25:1259–1264. https://doi.org/10.1249/00005768-199311000-00009
Lerchenmuller C, Rosenzweig A (2014) Mechanisms of exercise-induced cardiac growth. Drug Discov Today 19:1003–1009. https://doi.org/10.1016/j.drudis.2014.03.010
Li L, Muhlfeld C, Niemann B, Pan R, Li R, Hilfiker-Kleiner D, Chen Y, Rohrbach S (2011) Mitochondrial biogenesis and PGC-1alpha deacetylation by chronic treadmill exercise: differential response in cardiac and skeletal muscle. Basic Res Cardiol 106:1221–1234. https://doi.org/10.1007/s00395-011-0213-9
Loffredo FS, Nikolova AP, Pancoast JR, Lee RT (2014) Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res 115:97–107. https://doi.org/10.1161/CIRCRESAHA.115.302929
Mann N, Rosenzweig A (2012) Can exercise teach us how to treat heart disease? Circulation 126:2625–2635. https://doi.org/10.1161/CIRCULATIONAHA.111.060376
Moreira-Goncalves D, Henriques-Coelho T, Fonseca H, Ferreira RM, Amado F, Leite-Moreira A, Duarte JA (2011) Moderate exercise training provides left ventricular tolerance to acute pressure overload. Am J Physiol Heart Circ Physiol 300:H1044–H1052. https://doi.org/10.1152/ajpheart.01008.2010
Moreira-Goncalves D, Henriques-Coelho T, Fonseca H, Ferreira R, Padrao AI, Santa C, Vieira S, Silva AF, Amado F, Leite-Moreira A, Duarte JA (2015) Intermittent cardiac overload results in adaptive hypertrophy and provides protection against left ventricular acute pressure overload insult. J Physiol 593:3885–3897. https://doi.org/10.1113/JP270685
Moreira-Goncalves D, Ferreira R, Fonseca H, Padrao AI, Moreno N, Silva AF, Vasques-Novoa F, Goncalves N, Vieira S, Santos M, Amado F, Duarte JA, Leite-Moreira AF, Henriques-Coelho T (2015) Cardioprotective effects of early and late aerobic exercise training in experimental pulmonary arterial hypertension. Basic Res Cardiol 110:57. https://doi.org/10.1007/s00395-015-0514-5
Murphy E (2011) Estrogen signaling and cardiovascular disease. Circ Res 109:687–696. https://doi.org/10.1161/circresaha.110.236687
Naderi N, Hemmatinafar M, Gaeini AA, Bahramian A, Ghardashi-Afousi A, Kordi MR, Darbandi-Azar A, Karimzade F, Mohebbi H, Barati M (2019) High-intensity interval training increase GATA4, CITED4 and c-kit and decreases C/EBPβ in rats after myocardial infarction. Life Sci 221:319–326. https://doi.org/10.1016/j.lfs.2019.02.045
Nogueira-Ferreira R, Moreira-Goncalves D, Silva AF, Duarte JA, Leite-Moreira A, Ferreira R, Henriques-Coelho T (2016) Exercise preconditioning prevents MCT-induced right ventricle remodeling through the regulation of TNF superfamily cytokines. Int J Cardiol 203:858–866. https://doi.org/10.1016/j.ijcard.2015.11.066
Ozturk N, Olgar Y, Er H, Kucuk M, Ozdemir S (2017) Swimming exercise reverses aging-related contractile abnormalities of female heart by improving structural alterations. Cardiol J 24:85–93. https://doi.org/10.5603/CJ.a2016.0069
Padrão AI, Ferreira RMP, Vitorino R, Alves RMP, Neuparth MJ, Duarte JA, Amado F (2011) OXPHOS susceptibility to oxidative modifications: the role of heart mitochondrial subcellular location. Biochim Biophys Acta 1807:1106–1113. https://doi.org/10.1016/j.bbabio.2011.04.002
Powers SK, Smuder AJ, Kavazis AN, Quindry JC (2014) Mechanisms of exercise-induced cardioprotection. Physiology (Bethesda) 29:27–38. https://doi.org/10.1152/physiol.00030.2013
Sanz-de la Garza M, Rubies C, Batlle M, Bijnens BH, Mont L, Sitges M, Guasch E (2017) Severity of structural and functional right ventricular remodeling depends on training load in an experimental model of endurance exercise. Am J Physiol Heart Circ Physiol 313:H459–H468. https://doi.org/10.1152/ajpheart.00763.2016
Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W (2002) Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol 40:1856–1863. https://doi.org/10.1016/S0735-1097(02)02478-6
Sengupta P (2013) The laboratory rat: relating its age with human's. Int J Prev Med 4:624–630
Sicard D, Haak AJ, Choi KM, Craig AR, Fredenburgh LE, Tschumperlin DJ (2018) Aging and anatomical variations in lung tissue stiffness. Am J Physiol Lung Cell Mol Physiol 314:L946–L955. https://doi.org/10.1152/ajplung.00415.2017
Tiscornia GC, Moretta R, Argenziano MA, Amorena CE, Garcia Gras EA (2014) Inhibition of connexin 43 in cardiac muscle during intense physical exercise. Scand J Med Sci Sports 24:336–344. https://doi.org/10.1111/sms.12017
van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF (2000) Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192:1731–1744. https://doi.org/10.1084/jem.192.12.1731
Vettor R, Valerio A, Ragni M, Trevellin E, Granzotto M, Olivieri M, Tedesco L, Ruocco C, Fossati A, Fabris R, Serra R, Carruba MO, Nisoli E (2014) Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. Am J Physiol Endocrinol Metab 306:E519–E528. https://doi.org/10.1152/ajpendo.00617.2013
Wang Y, Wisloff U, Kemi OJ (2010) Animal models in the study of exercise-induced cardiac hypertrophy. Physiol Res 59:633–644
Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM (2014) The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J 35:2722–2731. https://doi.org/10.1093/eurheartj/ehs338
Wisloff U, Ellingsen O, Kemi OJ (2009) High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev 37:139–146. https://doi.org/10.1097/JES.0b013e3181aa65fc
Xiao J, Xu T, Li J, Lv D, Chen P, Zhou Q, Xu J (2014) Exercise-induced physiological hypertrophy initiates activation of cardiac progenitor cells. Int J Clin Exp Pathol 7:663–669
Acknowledgments
The authors would like to thank Celeste Resende for their assistance in sample preparation for morphological analysis.
Funding
This work was supported by the Portuguese Foundation for Science and Technology (FCT), European Union, QREN, and FEDER, and COMPETE funded the QOPNA research unit (project PEst-C/QUI/UI0062/2013), CIAFEL (UID/DTP/00617/2013), Unidade de Investigação Cardiovascular (UID/IC/00051/2013), iBiMED (UID/BIM/04501/2013), the research projects (EXPL/DTP-DES/1010/2013, FCOMP-01-0124-FEDER-041115, NETDIAMOND (SAICT-PAC/0047/2015)) and post-graduation students (grant numbers SFRH/BD/91067/2012 to R.N.F. and SFRH/BPD/90010/2012 to D.M.G.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The experiments were approved by the local Ethics Committee for Animal Experimentation (license number 008961) and performed in accordance with European Parliament Directive 2010/63/EU
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
Effect of exercise training on RAF-1 (74 kDa) in RV and LV and on RAF-1 and SIRT3 (28 kDa) content in isolated mitochondria. SED: sedentary, EX: exercise, LV: left ventricle, RV: right ventricle, RAF-1: RAF proto-oncogene serine/threonine-protein kinase, SIRT3: NAD-dependent deacetylase sirtuin-3. Representative immunoblots are shown above the correspondent graph (sample order has correspondence to the order of the groups presented in the graph). Values are presented as mean ± standard deviation (n = 4-6 per group). ***P < 0.001 vs. SED group. (PNG 175 kb)
Rights and permissions
About this article
Cite this article
Nogueira-Ferreira, R., Ferreira, R., Padrão, A.I. et al. One year of exercise training promotes distinct adaptations in right and left ventricle of female Sprague-Dawley rats. J Physiol Biochem 75, 561–572 (2019). https://doi.org/10.1007/s13105-019-00705-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-019-00705-4