Abstract
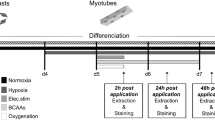
Hypoxia, occurring in several pathologies, has deleterious effects on skeletal muscle, in particular on protein homeostasis. Different induction methods of hypoxia are commonly used in cellular models to investigate the alterations of muscular function consecutive to hypoxic stress. However, a consensus is not clearly established concerning hypoxia induction methodology. Our aim was to compare oxygen deprivation with chemically induced hypoxia using cobalt chloride (CoCl2) or desferrioxamine (DFO) on C2C12 myotubes which were either cultured in hypoxia chamber at an oxygen level of 4% or treated with CoCl2 or DFO. For each method of hypoxia induction, we determined their impact on muscle cell morphology and on expression or activation status of key signaling proteins of synthesis and degradation pathways. The expression of HIF-1α increased whatever the method of hypoxia induction. Myotube diameter and protein content decreased exclusively for C2C12 myotubes submitted to physiological hypoxia (4% O2) or treated with CoCl2. Results were correlated with a hypophosphorylation of key proteins regulated synthesis pathway (Akt, GSK3-β and P70S6K). Similarly, the phosphorylation of FoxO1 decreased and the autophagy-related LC3-II was overexpressed with 4% O2 and CoCl2 conditions. Our results demonstrated that in vitro oxygen deprivation and the use of mimetic agent such as CoCl2, unlike DFO, induced similar responses on myotube morphology and atrophy/hypertrophy markers. Thus, physiological hypoxia or its artificial induction using CoCl2 can be used to understand finely the molecular changes in skeletal muscle cells and to evaluate new therapeutics for hypoxia-related muscle disorders.







Similar content being viewed by others
References
Caron MA, Thériault ME, Paré MÈ, Maltais F, Debigaré R (2009) Hypoxia alters contractile protein homeostasis in L6 myotubes. FEBS Lett 583(9):1528–1534. https://doi.org/10.1016/j.febslet.2009.04.006
Chaillou T, Lanner JT (2016) Regulation of myogenesis and skeletal muscle regeneration: effects of oxygen levels on satellite cell activity. FASEB J 30(12):3929–3941. https://doi.org/10.1096/fj.201600757R
Chen R, Jiang T, She Y, Xu J, Li C, Zhou S, Liu S (2017) Effects of cobalt chloride, a hypoxia-mimetic agent, on autophagy and atrophy in skeletal C2C12 myotubes. Biomed Res Int 2017. https://doi.org/10.1155/2017/7097580
Choy MK, Movassagh M, Bennett MR, Foo RS (2010) PKB/Akt activation inhibits p53-mediated HIF1A degradation that is independent of MDM2. J Cell Physiol 222(3):635–639. https://doi.org/10.1002/jcp.21980
Ciafrè SA, Niola F, Giorda E, Farace MG, Caporossi D (2007) CoCl2-simulated hypoxia in skeletal muscle cell lines: role of free radicals in gene up-regulation and induction of apoptosis. Free Radic Res 41(4):391–401. https://doi.org/10.1080/10715760601096799
Costes F, Gosker H, Feasson L, Desgeorges M, Kelders M, Castells J, Freyssenet D (2015) Impaired exercise training-induced muscle fiber hypertrophy and Akt/mTOR pathway activation in hypoxemic patients with COPD. J Appl Physiol 118(8):1040–1049. https://doi.org/10.1152/japplphysiol.00557.2014
de Theije CC, Langen RC, Lamers WH, Schols AM, Köhler SE (2013) Distinct responses of protein turnover regulatory pathways in hypoxia-and semistarvation-induced muscle atrophy. Am J Phys Lung Cell Mol Phys 305(1):L82–L91. https://doi.org/10.1152/ajplung.00354.2012
Debigaré R, Marquis K, Côté CH, Tremblay RR, Michaud A, LeBlanc P, Maltais F (2003) Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest 124(1):83–89. https://doi.org/10.1378/chest.124.1.83
Deldicque L, Francaux M (2013) Acute vs chronic hypoxia: what are the consequences for skeletal muscle mass? Cell Mol Exerc Physiol 2(1):e5. https://doi.org/10.7457/cmpe.2049-419
Di Carlo A, De Mori R, Martelli F, Pompilio G, Capogrossi MC, Germani A (2004) Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J Biol Chem 279(16):16332–16338. https://doi.org/10.1074/jbc.M313931200
Dziegala M, Kasztura M, Kobak K, Bania J, Banasiak W, Ponikowski P, Jankowska EA (2016) Influence of the availability of iron during hypoxia on the genes associated with apoptotic activity and local iron metabolism in rat H9C2 cardiomyocytes and L6G8C5 skeletal myocytes. Mol Med Rep 14(4):3969–3977. https://doi.org/10.3892/mmr.2016.5705
Egerman MA, Glass DJ (2014) Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 49(1):59–68. https://doi.org/10.3109/10409238.2013.857291
Favier FB, Costes F, Defour A, Bonnefoy R, Lefai E, Baugé S, Freyssenet D (2010) Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am J Phys Regul Integr Comp Phys 298(6):R1659–R1666. https://doi.org/10.1152/ajpregu.00550.2009
Guo Y, Gosker HR, Schols AM, Kapchinsky S, Bourbeau J, Sandri M, Hussain SN (2013) Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 188(11):1313–1320. https://doi.org/10.1164/rccm.201304-0732OC
Hayot M, Rodriguez J, Vernus B, Carnac G, Jean E, Allen D, Bonnieu A (2011) Myostatin up-regulation is associated with the skeletal muscle response to hypoxic stimuli. Mol Cell Endocrinol 332(1–2):38–47. https://doi.org/10.1016/j.mce.2010.09.008
Huang BW, Miyazawa M, Tsuji Y (2014) Distinct regulatory mechanisms of the human ferritin gene by hypoxia and hypoxia mimetic cobalt chloride at the transcriptional and post-transcriptional levels. Cell Signal 26(12):2702–2709. https://doi.org/10.1016/j.cellsig.2014.08.018
Ji W, Wang L, He S, Yan L, Li T, Wang J et al (2018) Effects of acute hypoxia exposure with different durations on activation of Nrf2-ARE pathway in mouse skeletal muscle. PLoS One 13(12):e0208474. https://doi.org/10.1371/journal.pone.0208474
Karovic O, Tonazzini I, Rebola N, Edström E, Lövdahl C, Fredholm BB, Daré E (2007) Toxic effects of cobalt in primary cultures of mouse astrocytes: similarities with hypoxia and role of HIF-1α. Biochem Pharmacol 73(5):694–708. https://doi.org/10.1016/j.bcp.2006.11.008
Kobak K, Kasztura M, Dziegala M, Bania J, Kapuśniak V, Banasiak W, Jankowska EA (2018) Iron limitation promotes the atrophy of skeletal myocytes, whereas iron supplementation prevents this process in the hypoxic conditions. Int J Mol Med 41(5):2678–2686. https://doi.org/10.3892/ijmm.2018.3481
Langen RC, Gosker HR, Remels AH, Schols AM (2013) Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int J Biochem Cell Biol 45(10):2245–2256. https://doi.org/10.1016/j.biocel.2013.06.015
Launay T, Hagström L, Lottin-Divoux S, Marchant D, Quidu P, Favret F, Beaudry M (2010) Blunting effect of hypoxia on the proliferation and differentiation of human primary and rat L6 myoblasts is not counteracted by Epo. Cell Prolif 43(1):1–8. https://doi.org/10.1111/j.1365-2184.2009.00648.x
Léger B, Cartoni R, Praz M, Lamon S, Dériaz O, Crettenand A, Russell AP (2006) Akt signalling through GSK-3β, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576(3):923–933. https://doi.org/10.1113/jphysiol.2006.116715
Li M, Tan J, Miao Y, Lei P, Zhang Q (2015) The dual role of autophagy under hypoxia-involvement of interaction between autophagy and apoptosis. Apoptosis 20(6):769–777. https://doi.org/10.1007/s10495-015-1110-8
Marabita M, Baraldo M, Solagna F, Ceelen JJ, Sartori R, Nolte H, Blaauw (2016) S6K1 is required for increasing skeletal muscle force during hypertrophy. Cell Rep 17(2):501–513. https://doi.org/10.1016/j.celrep.2016.09.020
Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F (2002) Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166(6):809–813. https://doi.org/10.1164/rccm.2107031
Martin NR, Aguilar-Agon K, Robinson GP, Player DJ, Turner MC, Myers SD, Lewis MP (2017) Hypoxia impairs muscle function and reduces myotube size in tissue engineered skeletal muscle. J Cell Biochem 118(9):2599–2605. https://doi.org/10.1002/jcb.25982
Pisani DF, Dechesne CA (2005) Skeletal muscle HIF-1α expression is dependent on muscle fiber type. J Gen Physiol 126(2):173–178. https://doi.org/10.1085/jgp.200509265
Pomiès P, Rodriguez J, Blaquière M, Sedraoui S, Gouzi F, Carnac G, Hayot M (2015) Reduced myotube diameter, atrophic signalling and elevated oxidative stress in cultured satellite cells from COPD patients. J Cell Mol Med 19(1):175–186. https://doi.org/10.1111/jcmm.12390
Remels AH, Gosker HR, Langen RC, Schols AM (2012) The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol 114(9):1253–1262. https://doi.org/10.1152/japplphysiol.00790.2012
Ren K, Crouzier T, Roy C, Picart C (2008) Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cell differentiation. Adv Funct Mater 18(9):1378–1389. https://doi.org/10.1002/adfm.200701297
Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG (2006) Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 571(2):415–424. https://doi.org/10.1113/jphysiol.2005.102327
Rovetta F, Stacchiotti A, Faggi F, Catalani S, Apostoli P, Fanzani A, Aleo MF (2013) Cobalt triggers necrotic cell death and atrophy in skeletal C2C12 myotubes. Toxicol Appl Pharmacol 271(2):196–205. https://doi.org/10.1016/j.taap.2013.05.005
Sandri M (2013) Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol 45(10):2121–2129. https://doi.org/10.1016/j.biocel.2013.04.023
Scaringi R, Piccoli M, Papini N, Cirillo F, Conforti E, Bergante S, Menicanti L (2013) NEU3 sialidase is activated under hypoxia and protects skeletal muscle cells from apoptosis through the activation of the epidermal growth factor receptor signaling pathway and the hypoxia-inducible factor (HIF)-1α. J Biol Chem 288(5):3153–3162. https://doi.org/10.1074/jbc.M112.404327
Zanchi NE, Lancha AH (2008) Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol 102(3):253–263. https://doi.org/10.1007/s00421-007-0588-3
Acknowledgements
We thank Amandine Girard and Matthias Lambert for their technical assistance.
Author information
Authors and Affiliations
Contributions
Samir Bensaid: Conceived, designed and performed the experiments, analysed the data and wrote the paper; Julie Fourneau: contributed to acquisition and analysis of the data; Claudine Fabre: conceived the experiments and wrote the paper; Caroline Cieniewski-Bernard: conceived and designed the experiments, performed the experiments, analysed the data and wrote the paper.
Corresponding author
Ethics declarations
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed consent
Not concerned.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bensaid, S., Fabre, C., Fourneau, J. et al. Impact of different methods of induction of cellular hypoxia: focus on protein homeostasis signaling pathways and morphology of C2C12 skeletal muscle cells differentiated into myotubes. J Physiol Biochem 75, 367–377 (2019). https://doi.org/10.1007/s13105-019-00687-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-019-00687-3