Abstract
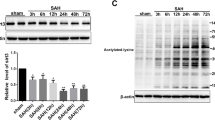
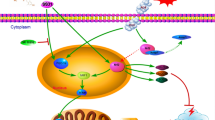
Neuronal injury following subarachnoid hemorrhage (SAH) has been shown to be associated with mitochondrial dysfunction and oxidative stress. βIIPKC, a subtype of protein kinase C (PKC), accumulates on the mitochondrial outer membrane and phosphorylates mitofusin 1 (Mfn1) at serine 86. Here, we investigated the role of Mfn1-βIIPKC interaction in brain damage and neurological function in both in vivo and in vitro experimental SAH models. The expression of βIIPKC protein and the interaction of Mfn1-βIIPKC were found to be increased after OxyHb treatment in primary cultured cortical neurons and were also observed in the brain following SAH in rats. Treatment with the βIIPKC inhibitor βIIV5-3 or SAMβA, a peptide that selectively antagonizes Mfn1-βIIPKC association, significantly attenuated the OxyHb-induced neuronal injury and apoptosis. These protective effects were accompanied by inhibited mitochondrial dysfunction and preserved mitochondrial biogenesis. The results of western blot showed that βIIV5-3 or SAMβA markedly increased the expression of Sirt3 and enhanced the activities of its downstream mitochondrial antioxidant enzymes in OxyHb-treated neurons. Knockdown of Sirt3 via specific targeted small interfering RNA (siRNA) partially prevented the βIIV5-3- or SAMβA-induced protection and antioxidative effects. In addition, treatment with βIIV5-3 or SAMβA in vivo was found to obviously reduce brain edema, alleviate neuroinflammation, and preserve neurological function after experimental SAH in rats. In congruent with in vitro data, the protection induced by βIIV5-3 or SAMβA was reduced by Sirt3 knockdown in vivo. In summary, our present results showed that blocking Mfn1-βIIPKC interaction protects against brain damage and mitochondrial dysfunction via Sirt3 following experimental SAH.









Similar content being viewed by others
References
Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2016;12(12):699–713.
Khey KMW, Huard A, Mahmoud SH. Inflammatory pathways following subarachnoid hemorrhage. Cell Mol Neurobiol. 2020;40(5):675–93.
Coulibaly AP, Provencio JJ. Aneurysmal subarachnoid hemorrhage: an overview of inflammation-induced cellular changes. Neurotherapeutics. 2020;17(2):436–45.
Callender JA, Newton AC. Conventional protein kinase C in the brain: 40 years later. Neuronal Signal. 2017;1(2):NS20160005.
Ono Y, et al. Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science. 1987;236(4805):1116–20.
Wakasaki H, et al. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci USA. 1997;94(17):9320–5.
Ferreira JC, Brum PC, Mochly-Rosen D. betaIIPKC and epsilonPKC isozymes as potential pharmacological targets in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51(4):479–84.
Saito N, et al. Distribution of protein kinase C-like immunoreactive neurons in rat brain. J Neurosci. 1988;8(2):369–82.
Mochly-Rosen D, Basbaum AI, Koshland DE Jr. Distinct cellular and regional localization of immunoreactive protein kinase C in rat brain. Proc Natl Acad Sci USA. 1987;84(13):4660–4.
Basu A, Pal D. Two faces of protein kinase Cdelta: the contrasting roles of PKCdelta in cell survival and cell death. ScientificWorldJournal. 2010;10:2272–84.
Tejero-Diez P, et al. bFGF stimulates GAP-43 phosphorylation at ser41 and modifies its intracellular localization in cultured hippocampal neurons. Mol Cell Neurosci. 2000;16(6):766–80.
Correas I, Diaz-Nido J, Avila J. Microtubule-associated protein tau is phosphorylated by protein kinase C on its tubulin binding domain. J Biol Chem. 1992;267(22):15721–8.
Yuan Z, Agarwal-Mawal A, Paudel HK. 14-3-3 binds to and mediates phosphorylation of microtubule-associated tau protein by Ser9-phosphorylated glycogen synthase kinase 3beta in the brain. J Biol Chem. 2004;279(25):26105–14.
Boehm J, et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51(2):213–25.
Ferreira JCB, et al. A selective inhibitor of mitofusin 1-betaIIPKC association improves heart failure outcome in rats. Nat Commun. 2019;10(1):329.
Chen T, et al. Down-regulation of Homer1b/c attenuates glutamate-mediated excitotoxicity through endoplasmic reticulum and mitochondria pathways in rat cortical neurons. Free Radic Biol Med. 2012;52(1):208–17.
Lu Y, et al. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J Neuroinflammation. 2018;15(1):87.
Laker RC, et al. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem. 2014;289(17):12005–15.
Ferreira JC, et al. Protein quality control disruption by PKCbetaII in heart failure; rescue by the selective PKCbetaII inhibitor, betaIIV5–3. PLoS One. 2012;7(3):e33175.
Chen Y, et al. Evaluation of the neuroprotective effect of EGCG: a potential mechanism of mitochondrial dysfunction and mitochondrial dynamics after subarachnoid hemorrhage. Food Funct. 2018;9(12):6349–59.
Zhang T, et al. Docosahexaenoic acid alleviates oxidative stress-based apoptosis via improving mitochondrial dynamics in early brain injury after subarachnoid Hemorrhage. Cell Mol Neurobiol. 2018;38(7):1413–23.
Simpson PC. Beta-protein kinase C and hypertrophic signaling in human heart failure. Circulation. 1999;99(3):334–7.
Patterson C, et al. The bitter end: the ubiquitin-proteasome system and cardiac dysfunction. Circulation. 2007;115(11):1456–63.
Roth BL, et al. Immunohistochemical distribution of beta-protein kinase C in rat hippocampus determined with an antibody against a synthetic peptide sequence. Brain Res Bull. 1989;22(5):893–7.
Sheu FS, et al. Glial-derived S100b protein selectively inhibits recombinant beta protein kinase C (PKC) phosphorylation of neuron-specific protein F1/GAP43. Brain Res Mol Brain Res. 1994;21(1–2):62–6.
Perez-Pinzon MA, Dave KR, Raval AP. Role of reactive oxygen species and protein kinase C in ischemic tolerance in the brain. Antioxid Redox Signal. 2005;7(9–10):1150–7.
Tanaka A, et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191(7):1367–80.
Leboucher GP, et al. Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell. 2012;47(4):547–57.
Chen L, et al. Molecular transporters for peptides: delivery of a cardioprotective epsilonPKC agonist peptide into cells and intact ischemic heart using a transport system, R(7). Chem Biol. 2001;8(12):1123–9.
Wang X, et al. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;109(Suppl 1):153–9.
Li Y, et al. Inhibition of mTOR alleviates early brain injury after subarachnoid hemorrhage via relieving excessive mitochondrial fission. Cell Mol Neurobiol. 2020;40(4):629–42.
Wu P, et al. Mdivi-1 alleviates early brain injury after experimental subarachnoid hemorrhage in rats, possibly via inhibition of Drp1-activated mitochondrial fission and oxidative stress. Neurochem Res. 2017;42(5):1449–58.
Thornton C, et al. Mitochondrial dynamics, mitophagy and biogenesis in neonatal hypoxic-ischaemic brain injury. FEBS Lett. 2018;592(5):812–30.
Chen H, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200.
Simmons EC, Scholpa NE, Schnellmann RG. Mitochondrial biogenesis as a therapeutic target for traumatic and neurodegenerative CNS diseases. Exp Neurol. 2020;329:113309.
Cameron RB, Beeson CC, Schnellmann RG. Development of therapeutics that induce mitochondrial biogenesis for the treatment of acute and chronic degenerative diseases. J Med Chem. 2016;59(23):10411–34.
Kang D, Hamasaki N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: overview of its multiple roles. Ann N Y Acad Sci. 2005;1042:101–8.
Ruszkiewicz J, Albrecht J. Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochem Int. 2015;88:66–72.
Anamika, et al. Mitochondrial SIRT3 and neurodegenerative brain disorders. J Chem Neuroanat. 2019;95:43–53.
Schlicker C, et al. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382(3):790–801.
Qiu X, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–7.
Dai SH, et al. Sirt3 confers protection against neuronal ischemia by inducing autophagy: Involvement of the AMPK-mTOR pathway. Free Radic Biol Med. 2017;108:345–53.
Dai SH, et al. Sirt3 attenuates hydrogen peroxide-induced oxidative stress through the preservation of mitochondrial function in HT22 cells. Int J Mol Med. 2014;34(4):1159–68.
Dai SH, et al. Sirt3 protects cortical neurons against oxidative stress via regulating mitochondrial Ca2+ and mitochondrial biogenesis. Int J Mol Sci. 2014;15(8):14591–609.
Chen T, et al. Sirt1-Sirt3 axis regulates human blood-brain barrier permeability in response to ischemia. Redox Biol. 2018;14:229–36.
Meng G, et al. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br J Pharmacol. 2018;175(8):1126–45.
Tyagi A, et al. SIRT3 deficiency-induced mitochondrial dysfunction and inflammasome formation in the brain. Sci Rep. 2018;8(1):17547.
Wu X, et al. SIRT3 protects against early brain injury following subarachnoid hemorrhage via promoting mitochondrial fusion in an AMPK dependent manner. Chin Neurosurg J. 2020;6:1.
Han H. RNA interference to knock down gene expression. Methods Mol Biol. 2018;1706:293–302.
Eschenbacher WH, et al. Two rare human mitofusin 2 mutations alter mitochondrial dynamics and induce retinal and cardiac pathology in Drosophila. PLoS One. 2012;7(9):e44296.
Kwon D, Park E, Kang SJ. Stimulator of IFN genes-mediated DNA-sensing pathway is suppressed by NLRP3 agonists and regulated by mitofusin 1 and TBC1D15, mitochondrial dynamics mediators. FASEB J. 2017;31(11):4866–78.
Ramirez S, et al. Mitochondrial dynamics mediated by mitofusin 1 is required for POMC neuron glucose-sensing and insulin release control. Cell Metab. 2017;25(6):1390-1399 e6.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81701932, No. 81871589, and No. 82072168), the Major Scientific Research Project of Wuxi Health Commission (No. Z202001), the Translational Medicine Research Major Project of Wuxi Health Commission (No. ZH201901), the Clinical Medical Science and Technology Development Foundation of Jiangsu University (No. JLY20180028), the China Postdoctoral Science Foundation funded project (No. 2019M651803), the Key Scientific Research Project of Jiangsu Health Commission (No. K2019018), and the Logistics Scientific Research Project of PLA (No. CLB20J027).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, T., Wang, Y., Wang, YH. et al. The Mfn1-βIIPKC Interaction Regulates Mitochondrial Dysfunction via Sirt3 Following Experimental Subarachnoid Hemorrhage. Transl. Stroke Res. 13, 845–857 (2022). https://doi.org/10.1007/s12975-022-00999-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-00999-5