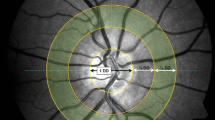
Abstract
Structural and functional similarities exist between the retinal, cerebral and, as previously suggested, the coronary microvasculature. Retinal microvascular structure and functionality (in response to flicker-light-induced-provocation (FLIP)) may relate to coronary artery disease risk and possible stroke risk. We investigated associations between retinal vessel structure, functionality and cardiac stress markers (cardiac troponin T [cTnT], amino-terminal B-type natriuretic peptide [NT-proBNP]) to translate these retina–heart relationships to stroke risk. We included 317 African and Caucasian teachers’ (aged 23–68 years), who participated in the Sympathetic Activity and Ambulatory Blood Pressure in Africans (SABPA) study. Fasting plasma and serum samples for cTnT and NT-proBNP were collected. Retinal vascular calibres were quantified from fundus images and dynamic retinal vessel calibre responses during FLIP. The University of California stroke risk score was applied to assess sub-clinical 10-year stroke risk. cTnT levels were similar in Africans and Caucasians, whereas NT-proBNP levels were lower in Africans. In Africans, a reduced arteriolar calibre and attenuated arteriolar dilation during FLIP was associated with higher cTnT (p < 0.01). Their larger retinal–venular calibre (p < 0.02) and attenuated arteriolar dilation during FLIP (p < 0.05) were associated with lower NT-proBNP. Again, exclusively in Africans, increased cardiac stress, wider venular calibres and retinal arteriovenous nicking predicted an increased 10-year stroke risk with odds ratios of 1.57 (95% CI, 1.34; 1.68, p = 0.031), 1.51 (95% CI, 1.26; 1.59, p = 0.002), 1.10 (95% CI, 0.94; 2.85, p = 0.002) and 1.06 (95% CI 0.83; 1.56, p = 0.052), respectively. None of these associations were evident in the Caucasian group. Investigating the retinal vasculature may serve as a tool to approximate sub-clinical coronary and cerebral microvasculature damage or dysfunction. These cardiac stress–retinal associations additionally predicted a greater stroke risk in the SABPA African cohort. Observable changes in the retinal vasculature may serve as markers for the identification and prediction of cardio-systemic and cerebral vascular morbidities and risks, thereby establishing a brain-heart link.

Proposed series of events during which sustained high pressure and increased cardiac stress may alter retinal reactivity and link to increased stroke risk

Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- DVA:
-
Dynamic vessel analyses
- cTnT:
-
Cardiac troponin T
- MC:
-
Maximum constriction
- MD:
-
Maximum dilation
- NT-proBNP:
-
Amino-terminal pro-B-type natriuretic peptide
- OR:
-
Odds ratio
References
Rousso L, Sowka J. Recognizing abnormal vasculature: a guide to following and educating patients who face this class of sight-threatening diagnosis. Rev Opthalmol 2017.
Kur J, Newman EA, Chang-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina choroid in health disease. Prog Retin Eye Res. 2012;31(5):377–406.
Chang-Ling T. Blood-brain barriers, Wiley-VCH Verlag, GmbH & Co, KgaA:2006. The blood retina interface similarities and contrasts with the blood-brain interface: 701–724.
Terai N, Spoerl E, Pillunat LE, Stodtmeister R. The effect of caffeine on retinal vessel diameter in young healthy subjects. Acta Opthalmol. 2012;90:524–8.
Al-Fiahd AH, Farouque O, Kawasaki R, Nguyen TT, Uddin N, Freeman M, et al. Retinal microvascular structure and function in patients with risk factors of atherosclerosis and coronary artery disease. Atherosclerosis. 2014;233:478–84.
Ong YT, Wong TY, Klein R, Klein BE, Mithcell P, Sharrett AR. Hypertensive retinopathy and risk of stroke. Hypertension. 2013;62(4):706–11.
Patton N, Aslam T, MacGillivray T, Pattie A, Deary IJ, Dhillon R. Retinal vascular image analysis a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–48.
Moss HE. Retinal vascular changes are a marker for cerebral vascular diseases. Curr Neurol Neurosci Rep. 2015;15(7):1–15.
Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD. Retinal microvasculature abnormalities and incident stroke. The Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–40.
Mutlu U, Ikram MA, Hofman A, de Jong PTVM, Klaver CCW, Ikram MK. N-terminal pro-B-type natriuretic peptide is related to retinal microvascular damage: the Rotterdam study. Arterioscler Thromb Vasc Biol. 2016;36:1698–702.
Koerbin GL. High sensitivity troponin T—its use in diagnosis of cardiac dysfunction. Thesis for fulfillment of PhD, University of Canberra, Australia, 2014.
White HD. Pathophysiology of troponin elevation: do elevation occur with myocardial infarction as well as necrosis? J Am Coll Cardiol. 2011;57:2406–8.
Schneider ALC, Rawlings AM, Sharrett AR, Alonso A, Mosley TH, Hoogeveen RC, et al. High-sensitivity cardiac troponin T and cognitive function and dementia risk: the atherosclerosis risk in communities study. EHJ. 2014;35:1817–24.
Moreno V, Hernandez-Romero D, Volchez JA, Garcia-Honrubia A, Cambronero F, Casas T, et al. Serum levels of high-sensitivity troponin T: a novel marker for cardiac remodelling in hypertrophic cardiomyopathy. J Card Fail. 2010;16:950–6.
Reddy K, Khaliq A, Henning RJ. Recent advances in the diagnosis and treatment of acute myocardial infarction. WJC. 7(5):243–76.
Van Vuren EJ, Malan L, von Känel R, Cockeran M, Malan NT. Hyperpulsatile pressure, systemic inflammation and cardiac stress are associated with cardiac wall remodeling in an African male cohort: the SABPA study. Hypertens Res. 2016;39(9):648–53.
Ravassa S, Kuznetsova T, Varo N, Thijs L, Delles C, Dominiczak A, et al. Biomarkers of cardiomyocyte injury and stress identify left atrial and left ventricular remodelling and dysfunction: a population-based study. Int J Cardiol. 2015;185:177–85.
Aaltonen V, Kinnunen K, Jouhilahti E, Peltonen J, Nikinmaa M, Kaarniranta K, et al. Hypoxic conditions stimulate the release of B-type natriuretic peptide from human retinal pigment epithelium cell culture. Acta Opthalmol. 2014;92:740–4.
Prado J, Baltrons MA, Pifarré GA. Glial cells as sources and targets of natriuretic peptides. Neurochem Int. 2010;57:367–74.
Reinhard H, Wiinberg N, Hansen PR, Kjaer A, Petersen CL, Winther K, et al. NT-proBNP levels, atherosclerosis and vascular function in asymptomatic type 2 diabetic patients. Cardiovasc Diab. 2011;10:71.
Cushman M, Judd SE, Howard VJ, Kissela B, Gutiérrez OM, Jenny NS, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the Reasons for Geographic And Racial Differences in Stroke cohort. Stroke. 2014;45(6):1646–50.
Malan L, Hamer M, von Känel R, Schlaich MP, Riemann M, Malan NT, et al. Chronic depression symptoms and salivary NOx are associated with retinal vascular dysregulation: the SABPA study. Biol Chem. 2016:55–6.
Malan NT, von Känel R, Smith W, Lambert GW, Vilser W, Eikelis N et al. A challenged sympathetic system is associated with retinal calibre in a black male cohort: The SABPA study. Microc Health Dis 2016, prov chapter.
Croghan C, Egeghy PP. Methods for dealing with values below the limit of detection using SAS. Presented sat Southern SAS user group, St Petersburg, FL, September 22-24, 2003.
Malan L, Hamer M, von Känel R, Lambert GW, Delport R, Steyn HS, et al. Chronic defensiveness and neuroendocrine dysfunction reflect a novel cardiac troponin T cut point: the SABPA study. Psychoneuroendocrin. 2017;28(85):30–27.
Griffiths ME, Delport R, Riemann M, Malan L. Lower high-sensitivity cardiac troponin T cut-points in blacks to predict hypertension: the SABPA study. Eur J Prev Cardiol. 2017;24(9):942–50.
Januzzi JL, van Kimmenade R, Lainchbury J. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1,256 patients; the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330–7.
Marina N, Teschemacher AG, Kasparov S, Gourine AV. Glia, sympathetic activity and cardiovascular disease. Exp Physiol. 2016;101:565–76.
Henderson AD, Bruce BB, Newman NJ, Biousse V. Hypertension-related eye abnormalities and the risk for stroke. Rev Neurol Dis. 2011;8(1–2):1–9.
Wong TY, Islam FMA, Klein R, Klein B, Cotch MF, Castro C, et al. Retinal vascular calibre, cardiovascular risk factors and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Opthalmol Vis Sci. 2006;47(6):2341–50.
McGeecan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BEK, et al. Retinal vessel calibre and risk for coronary heart disease: a systematic review and meta-analysis. Ann Intern Med. 2009;151(6):404–13.
Vafaie M, Giannitsis E, Mueller-Hennessen M, Biener M, Gorochow E, Katus HA et al. High-sensitivity cardiac troponin T and stroke risk in patients admitted to an emergency department with atrial fibrillation. EHJ 2017; 38: suppl_1.
Liu J, Wang D, Xiong Y, Liu B, Wei C, Ma Z, et al. High-sensitivity cardiac troponin T and risk of cerebral microbleeds in acute ischemic stroke patients with atrial fibrillation and/or rheumatic heart disease. J Neurol Sci. 2016;369:15–8.
Malan L, Hamer M, Schlaich MP, Lambert GW, Ziemssen T, Malan NT, et al. Defensive coping facilitates higher blood pressure and early sub-clinical structural vascular disease via alterations in heart rate variability: the SABPA study. Atherosclerosis. 2013;227:391–7.
Wentzel A, Malan L, Scheepers JD, Malan NT. QTc prolongation, increased NT-proBNP and pre-clinical myocardial wall remodelling in excessive alcohol consumers: the SABPA study. Alcohol. 2018 (in press);68:1–8.
Behnke BJ, Zawieja DC, Gashev AA, Ray CA, Delp MD. Diminished mesenteric vaso-and venoconstriction and elevated plasma ANP and BNP with simulated microgravity. J Appl Physiol. 2008;104:1273–80.
Takashio S, Yamamuro M, Izumiya Y, Sugiyama S, Kojima S, Yamamoto E, et al. Coronary microvascular dysfunction and diastolic load correlate with cardiac troponin T release measured by a high-sensitivity assay in patients with nonischemic heart failure. JACC. 2013;62(7):632–40.
Conzen C, Albanna W, Weiss M, Kürten D, Vilser W, Kotliar K, et al. Vasoconstriction and impairment of neurovascular coupling after subarachnoid haemorrhage: a descriptive analysis of retinal changes. Transl Stroke Res. 2017. https://doi.org/10.1007/s12975-017-0585-8.
Funding
The present study was partially funded by the National Research Foundation, South African Medical Research Council, ROCHE Diagnostics, North-West University (Potchefstroom Campus), North-West Department of Education South Africa as well as the Metabolic Syndrome institute, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The SABPA study obtained ethical approval from the Health Research Ethics Committee (HREC) of the NWU and extended approval was granted for the second phase (ethics number: NWU-00036-07-S6). Written informed consent was obtained from all volunteers prior to participation. All procedures and objectives were explained to the participants prior to their recruitment and adhered to the applicable institutional guidelines and terms, as stated by the Declaration of Helsinki (2004). Please also refer to uploaded SABPA Protocol article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimers
None.
Additional information
Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors; therefore, funders do not accept any liability regarding this study.
Electronic Supplementary Material
ESM 1
(DOCX 85 kb)
Rights and permissions
About this article
Cite this article
Wentzel, A., Malan, L., Smith, W. et al. Retinal Vasculature Reactivity During Flicker Light Provocation, Cardiac Stress and Stroke Risk in Africans: The SABPA Study. Transl. Stroke Res. 10, 485–494 (2019). https://doi.org/10.1007/s12975-018-0673-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0673-4