Abstract
Neurovascular unit (NVU) is considered as a conceptual framework for investigating the mechanisms as well as developing therapeutic targets for ischemic and hemorrhagic stroke. From a molecular perspective, oxidative stress, excitotoxicity, inflammation, and disruption of the blood brain barrier are broad pathophysiological frameworks on the basis on which potential therapeutic candidates for ischemic and hemorrhagic stroke could be discussed. Cofilin is a potent actin-binding protein that severs and depolymerizes actin filaments in order to generate the dynamics of the actin cytoskeleton. Although studies of the molecular mechanisms of cofilin-induced reorganization of the actin cytoskeleton have been ongoing for decades, the multicellular functions of cofilin and its regulation in different molecular pathways are expanding beyond its primary role in actin cytoskeleton. This review focuses on the role of cofilin in oxidative stress, excitotoxicity, inflammation, and disruption of the blood brain barrier in the context of NVU as well as how and why cofilin could be studied further as a potential target for ischemic and hemorrhagic stroke.
Similar content being viewed by others
References
Mozaffarian D et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322.
Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–98.
Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–7.
Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. 2009;22(3):294–301.
Kitagawa K. CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J. 2007;274(13):3210–7.
Terasaki Y et al. Mechanisms of neurovascular dysfunction in acute ischemic brain. Curr Med Chem. 2014;21(18):2035–42.
Lok J et al. Intracranial hemorrhage: mechanisms of secondary brain injury. Acta Neurochir Suppl. 2011;111:63–9.
Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781–6.
Lyden P et al. Clomethiazole Acute Stroke Study in ischemic stroke (CLASS-I): final results. Stroke. 2002;33(1):122–8.
Shuaib A et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357(6):562–71.
Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415.
Gladstone DJ et al. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33(8):2123–36.
Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068.
Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8(1):82–9.
Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010;1207:50–7.
Van Troys M et al. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87(8–9):649–67.
Bellenchi GC et al. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev. 2007;21(18):2347–57.
Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24(1):13–23.
Chan C, Beltzner CC, Pollard TD. Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr Biol. 2009;19(7):537–45.
Niwa R et al. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108(2):233–46.
Ambach A et al. The serine phosphatases PP1 and PP2A associate with and activate the actin-binding protein cofilin in human T lymphocytes. Eur J Immunol. 2000;30(12):3422–31.
Gohla A, Birkenfeld J, Bokoch GM. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7(1):21–9.
Kim JS, Huang TY, Bokoch GM. Reactive oxygen species regulate a slingshot-cofilin activation pathway. Mol Biol Cell. 2009;20(11):2650–60.
Wang Y, Shibasaki F, Mizuno K. Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J Biol Chem. 2005;280(13):12683–9.
Huang TY et al. Chronophin mediates an ATP-sensing mechanism for cofilin dephosphorylation and neuronal cofilin-actin rod formation. Dev Cell. 2008;15(5):691–703.
Park S, Jung Y. Combined actions of Na/K-ATPase, NCX1 and glutamate dependent NMDA receptors in ischemic rat brain penumbra. Anat Cell Biol. 2010;43(3):201–10.
Bamburg JR et al. ADF/cofilin-actin rods in neurodegenerative diseases. Curr Alzheimer Res. 2010;7(3):241–50.
Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230.
Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006;18(1):26–31.
Bailly M, Jones GE. Polarised migration: cofilin holds the front. Curr Biol. 2003;13(4):R128–30.
Nusco GA et al. Modulation of calcium signalling by the actin-binding protein cofilin. Biochem Biophys Res Commun. 2006;348(1):109–14.
Ohashi K. Roles of cofilin in development and its mechanisms of regulation. Dev Growth Differ. 2015;57(4):275–90.
Schonhofen P et al. Cofilin/actin rod formation by dysregulation of cofilin-1 activity as a central initial step in neurodegeneration. Mini Rev Med Chem. 2014;14(5):393–400.
Maki T et al. Biphasic mechanisms of neurovascular unit injury and protection in CNS diseases. CNS Neurol Disord Drug Targets. 2013;12(3):302–15.
Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59(5):735–42.
Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11(4):369–80.
Kriz J, Lalancette-Hebert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117(5):497–509.
Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72.
Wolf M et al. ADF/cofilin controls synaptic actin dynamics and regulates synaptic vesicle mobilization and exocytosis. Cereb Cortex. 2015;25(9):2863–75.
Gu J et al. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13(10):1208–15.
Yuen EY et al. Regulation of AMPA receptor channels and synaptic plasticity by cofilin phosphatase Slingshot in cortical neurons. J Physiol. 2010;588(Pt 13):2361–71.
Rust MB et al. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. EMBO J. 2010;29(11):1889–902.
Arvidsson A et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70.
Zhang RL et al. Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse. J Cereb Blood Flow Metab. 2009;29(7):1240–50.
Nawaz S et al. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev Cell. 2015;34(2):139–51.
Pedraza, C.E., et al., Induction of oligodendrocyte differentiation and in vitro myelination by inhibition of rho-associated kinase. ASN Neuro, 2014. 6(4).
Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808.
Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Exp Neurol. 2010;225(1):24–8.
Gitik M et al. Phagocytic receptors activate and immune inhibitory receptor SIRPalpha inhibits phagocytosis through paxillin and cofilin. Front Cell Neurosci. 2014;8:104.
Cao W et al. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett. 1988;88(2):233–8.
Floyd RA et al. Translational research involving oxidative stress and diseases of aging. Free Radic Biol Med. 2011;51(5):931–41.
Gouriou Y et al. Mitochondrial calcium handling during ischemia-induced cell death in neurons. Biochimie. 2011;93(12):2060–7.
Zhang Y et al. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J Neurosci. 2004;24(47):10616–27.
Klamt F et al. Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nat Cell Biol. 2009;11(10):1241–6.
Wabnitz GH et al. Mitochondrial translocation of oxidized cofilin induces caspase-independent necrotic-like programmed cell death of T cells. Cell Death Dis. 2010;1:e58.
Madineni, A., Q. Alhadidi, and Z.A. Shah, Cofilin inhibition restores neuronal cell death in oxygen-glucose deprivation model of ischemia. Mol Neurobiol, 2015. doi:10.1007/s12035-014-9056-3.
Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14(5):497–500.
Hayakawa K et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31(29):10666–70.
Cavaliere F et al. Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell Death Dis. 2012;3:e268.
Fleissner F, Thum T. Critical role of the nitric oxide/reactive oxygen species balance in endothelial progenitor dysfunction. Antioxid Redox Signal. 2011;15(4):933–48.
Miller AA, Drummond GR, Sobey CG. Novel isoforms of NADPH-oxidase in cerebral vascular control. Pharmacol Ther. 2006;111(3):928–48.
Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173(1):2–13.
Lee MY et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29(4):480–7.
Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22(4):371–8.
Mronga T et al. Mitochondrial pathway is involved in hydrogen-peroxide-induced apoptotic cell death of oligodendrocytes. Glia. 2004;46(4):446–55.
Lindenau J et al. Cellular distribution of superoxide dismutases in the rat CNS. Glia. 2000;29(1):25–34.
Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267(16):4912–6.
Chen Y et al. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem. 2001;77(6):1601–10.
Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15(11):1382–402.
Mabuchi T et al. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. J Neurosci. 2001;21(23):9204–13.
Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7(2):357–68.
Kostandy BB. The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol Sci. 2012;33(2):223–37.
Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69(4):369–81.
Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403(6767):316–21.
Posadas I et al. Cofilin activation mediates Bax translocation to mitochondria during excitotoxic neuronal death. J Neurochem. 2012;120(4):515–27.
Chen B et al. Both NMDA and non-NMDA receptors mediate glutamate stimulation induced cofilin rod formation in cultured hippocampal neurons. Brain Res. 2012;1486:1–13.
Sutherland BA et al. Cerebral blood flow alteration in neuroprotection following cerebral ischaemia. J Physiol. 2011;589(Pt 17):4105–14.
Duffney LJ et al. Shank3 deficiency induces NMDA receptor hypofunction via an actin-dependent mechanism. J Neurosci. 2013;33(40):15767–78.
Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14.
Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195(4284):1356–8.
Chaudhry FA et al. Glutamine uptake by neurons: interaction of protons with system a transporters. J Neurosci. 2002;22(1):62–72.
Kvamme E, Roberg B, Torgner IA. Phosphate-activated glutaminase and mitochondrial glutamine transport in the brain. Neurochem Res. 2000;25(9–10):1407–19.
McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85(15):3347–58.
Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105.
Adolph O et al. Rapid increase of glial glutamate uptake via blockade of the protein kinase A pathway. Glia. 2007;55(16):1699–707.
Sheean RK et al. Links between L-glutamate transporters, Na+/K + −ATPase and cytoskeleton in astrocytes: evidence following inhibition with rottlerin. Neuroscience. 2013;254:335–46.
Yan X et al. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014;62(7):1093–109.
Funfschilling U et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–21.
Wilkins A et al. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23(12):4967–74.
Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23(3):263–74.
Karadottir R et al. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162–6.
Yoshioka A et al. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors mediate excitotoxicity in the oligodendroglial lineage. J Neurochem. 1995;64(6):2442–8.
Simonishvili S et al. Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia-ischemia. ASN Neuro. 2013;5(5):e00131.
Mathur BN, Deutch AY. Rat meningeal and brain microvasculature pericytes co-express the vesicular glutamate transporters 2 and 3. Neurosci Lett. 2008;435(2):90–4.
Sharp CD et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am J Physiol Heart Circ Physiol. 2003;285(6):H2592–8.
Basuroy S, Leffler CW, Parfenova H. CORM-A1 prevents blood-brain barrier dysfunction caused by ionotropic glutamate receptor-mediated endothelial oxidative stress and apoptosis. Am J Physiol Cell Physiol. 2013;304(11):C1105–15.
Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69.
Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34.
Takano T et al. Astrocytes and ischemic injury. Stroke. 2009;40(3 Suppl):S8–12.
Barreto GE et al. Astrocyte proliferation following stroke in the mouse depends on distance from the infarct. PLoS One. 2011;6(11):e27881.
Wagner KR. Modeling intracerebral hemorrhage: glutamate, nuclear factor-kappa B signaling and cytokines. Stroke. 2007;38(2 Suppl):753–8.
Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92(4):463–77.
Luo XG, Chen SD. The changing phenotype of microglia from homeostasis to disease. Transl Neurodegener. 2012;1(1):9.
Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–89.
Zhou Y et al. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44.
Lee Y et al. Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int. 2014;2014:297241.
Jin R et al. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013;6(5):834–51.
Jonsson F et al. Immunological responses and actin dynamics in macrophages are controlled by N-cofilin but are independent from ADF. PLoS One. 2012;7(4):e36034.
Li J et al. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol. 2007;9(3):276–86.
Rasmussen I et al. Effects of F/G-actin ratio and actin turn-over rate on NADPH oxidase activity in microglia. BMC Immunol. 2010;11:44.
Hadas S et al. Complement receptor-3 negatively regulates the phagocytosis of degenerated myelin through tyrosine kinase Syk and cofilin. J Neuroinflammation. 2012;9:166.
Fu R et al. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol. 2014;49(3):1422–34.
Walsh KP et al. Amyloid-beta and proinflammatory cytokines utilize a prion protein-dependent pathway to activate NADPH oxidase and induce cofilin-actin rods in hippocampal neurons. PLoS One. 2014;9(4):e95995.
Engelhardt S, Patkar S, Ogunshola OO. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol. 2014;171(5):1210–30.
Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130(1):55–70.
Structure and function of the blood-brain barrier. 2010. 37(1): p. 13–25.
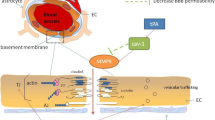
Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–8.
Keep RF et al. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18.
Koto T et al. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170(4):1389–97.
Bauer AT et al. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab. 2010;30(4):837–48.
Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cereb Blood Flow Metab. 2010;30(11):1847–59.
Kondo N et al. Thrombin induces rapid disassembly of claudin-5 from the tight junction of endothelial cells. Exp Cell Res. 2009;315(17):2879–87.
Moller T, Weinstein JR, Hanisch UK. Activation of microglial cells by thrombin: past, present, and future. Semin Thromb Hemost. 2006;32 Suppl 1:69–76.
Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27(5):894–908.
Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77(1):53–63.
Engelhardt S et al. Differential responses of blood-brain barrier associated cells to hypoxia and ischemia: a comparative study. Fluids Barriers CNS. 2015;12:4.
Liu LB et al. Bradykinin increases blood-tumor barrier permeability by down-regulating the expression levels of ZO-1, occludin, and claudin-5 and rearranging actin cytoskeleton. J Neurosci Res. 2008;86(5):1153–68.
Suurna MV et al. Cofilin mediates ATP depletion-induced endothelial cell actin alterations. Am J Physiol Renal Physiol. 2006;290(6):F1398–407.
Toshima J et al. Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in integrin-mediated actin reorganization and focal adhesion formation. Mol Biol Cell. 2001;12(4):1131–45.
Kobayashi M et al. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J. 2006;25(4):713–26.
Nagumo Y et al. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem Biophys Res Commun. 2008;377(3):921–5.
Shiobara T et al. The reversible increase in tight junction permeability induced by capsaicin is mediated via cofilin-actin cytoskeletal dynamics and decreased level of occludin. PLoS One. 2013;8(11):e79954.
Leonard A et al. Thrombin selectively engages LIM kinase 1 and slingshot-1L phosphatase to regulate NF-kappaB activation and endothelial cell inflammation. Am J Physiol Lung Cell Mol Physiol. 2013;305(9):L651–64.
Tominaga N et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Qasim Alhadidi is supported by the Higher Committee for Education Development in Iraq and Muhammad Shahdaat Bin Sayeed is supported by the Fulbright Program sponsored by the U.S. Department of State’s Bureau of Educational and Cultural Affairs.
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
No animals were used in this study.
Additional information
Qasim Alhadidi and Muhammad Shahdaat Bin Sayeed contributed equally to this work.
Rights and permissions
About this article
Cite this article
Alhadidi, Q., Bin Sayeed, M.S. & Shah, Z.A. Cofilin as a Promising Therapeutic Target for Ischemic and Hemorrhagic Stroke. Transl. Stroke Res. 7, 33–41 (2016). https://doi.org/10.1007/s12975-015-0438-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-015-0438-2