Abstract
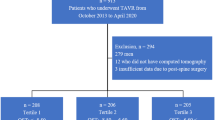
Low body weight and advanced age are among the best predictors of osteoporosis. Osteoporosis Self-Assessment Tool (OST) values are calculated by a simple formula [(body weight in kilograms – age in years) × 0.2] to identify patients at increased risk of osteoporosis. In our recent single-center study, we demonstrated an association between OST and poor outcomes in postmenopausal women after transcatheter aortic valve replacement (TAVR). We aimed to investigate the impact of osteoporotic risk in men with aortic stenosis who underwent TAVR in a large cohort. In this multi-center study, 1,339 men who underwent TAVR between April 2010 and July 2023 were retrospectively analyzed. Women were excluded from the present study. All patients were deemed appropriate for TAVR after a review by a multidisciplinary team. Baseline characteristics of patients were compared by dividing patients into three tertiles, based on the OST value: ≤ − 6.16, − 6.16 to − 4.14, and − 4.14 < for tertiles 1, 2, and 3, respectively. Primary endpoint was all-cause mortality after TAVR. Tertile 1 (patients with the lowest OST values) included older patients with smaller body mass index, lower hemoglobin and albumin levels. In addition, they had greater clinical frailty scale, slower 5-meter walk test, weaker hand grip strength, and more cognitive impairment, indicating increased frailty. They were more severely symptomatic, with lower ejection fractions, smaller aortic valve areas, and more tricuspid regurgitation than were patients in the other two groups. Multivariate analysis revealed that OST tertiles 3 was associated with decreased risk of all-cause mortality (hazard ratio, 0.66; 95% confidence interval, 0.48–0.90), compared with OST tertile 1 as a reference. For OST tertiles 1, 2, and 3, the estimated 1-year survival rates of all-cause mortality post-TAVR were 83.6% ± 1.9%, 91.1% ± 1.4%, and 93.1% ± 1.3%, respectively, (log-rank, p < 0.001). In conclusions, in men as same as women, osteoporotic risk assessed by OST values was overlapped with increased frailty. The simple OST formula was useful for predicting all-cause mortality in patients undergoing TAVR in large registry datasets.
Graphical Abstract




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author [MS], upon reasonable request.
Abbreviations
- AS:
-
Aortic stenosis
- DXA:
-
Dual-energy x-ray absorptiometry
- OST:
-
Osteoporosis self-assessment tool
- TAVR:
-
Transcatheter aortic valve replacement
References
Peck WA. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–50.
Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the national osteoporosis risk assessment. JAMA. 2001;286:2815–22.
Koh LKH, Sedrine WB, Torralba TP, et al. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12:699–705.
Dargent-Molina P, Poitiers F, Breart G, EPIDOS Group. In elderly women weight is the best predictor of a very low bone mineral density: evidence from the EPIDOS study. Osteoporos Int. 2000;11:881–8.
Chang AJ, Ying Q, Chen XN, Wang WM, Chen N. Evaluation of three risk assessment tools in discriminating fracture status among Chinese patients undergoing hemodialysis. Osteoporos Int. 2016;27:3599–606.
Logan S, Thu WPP, Lay WK, Wang LY, Cauley JA, Yong EL. Chronic joint pain and handgrip strength correlates with osteoporosis in mid-life women: a Singaporean cohort. Osteoporos Int. 2017;28:2633–43.
Subramaniam S, Chan CY, Soelaiman IN, et al. The performance of osteoporosis self-assessment tool for Asians (OSTA) in identifying the risk of osteoporosis among Malaysian population aged 40 years and above. Arch Osteoporos. 2019;14:117.
Leon MB, Smith CR, Mack M, PARTNER Trial Investigators, et al. Transcatheter aortic valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607.
Saji M, Higuchi R, Izumi Y, et al. Prevalence and impact of fracture on postmenopausal women with aortic stenosis who underwent transcatheter aortic valve replacement. Cardiovasc Interv Ther. 2022;37:543–8.
Saji M, Nanasato M, Higuchi R, et al. Impact of osteoporotic risk in women undergoing transcatheter aortic valve replacement. Cardiovasc Interv Ther. 2023. https://doi.org/10.1007/s12928-023-00940-z.
Chieffo A, Petronio AS, Mehilli J, et al. Acute and 30-day outcomes in women after TAVR: results from the WIN-TAVI (women’s INternational transcatheter aortic valve implantation) real-world registry. JACC Cardiovasc Interv. 2016;9:1589–600.
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. JAMA. 2018;319:2521–31.
Yokoyama H, Tobaru T, Muto Y, et al. Long-term outcomes in Japanese nonagenarians undergoing transcatheter aortic valve implantation: a multi-center analysis. Clin Cardiol. 2019;42:605–11.
Nishida K, Saji M, Higuchi R, et al. Predictors for all-cause mortality in men after transcatheter aortic valve replacement: a report from the LAPLACE-TAVI Registry. Int J Cardiol Heart Vasc. 2023;48:101257.
Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30:303–71.
Shimura T, Yamamoto M, Kano S, et al. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation. 2017;135:2013–24.
Saji M, Tobaru T, Higuchi R, et al. Cognitive assessment using the revised Hasegawa’s dementia scale to determine the mid-term outcomes following transcatheter aortic valve replacement. J Cardiol. 2019;74:206–11.
Fried LP, Tangen CM, Walston J, Cardiovascular Health Study Collaborative Research Group, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56.
Huemer MT, Kluttig A, Fischer B, et al. Grip strength values and cut-off points based on over 200,000 adults of the German National Cohort—a comparison to the EWGSOP2 cut-off points. Age Ageing. 2023;2023(52):afac324.
Prasad D, Nguyen MH. Chronic hepatitis, osteoporosis, and men: under-recognised and underdiagnosed. Lancet Diabetes Endocrinol. 2021;9:141.
Vilaca T, Eastell R, Schini M. Osteoporosis in men. Lancet Diabetes Endocrinol. 2022;10:273–83.
Gielen E, Dupont J, Dejaeger M, Laurent MR. Sarcopenia, osteoporosis and frailty. Metabolism. 2023;145:155638.
Compston JE, McClung MR, Leslie WD. Osteoporosis Lancet. 2019;393:364–76.
Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. J Am Coll Cardiol. 2015;65:976–83.
Acknowledgements
The authors thank all members of the Structural Heart Team of, Yamagata University, Mie University, Hirosaki University, Juntendo University, Kawasaki Saiwai Hospital and the Sakakibara Heart Institute.
Funding
This study was supported by a research grant from Sakakibara Internal Research Grant for Promotion of Sciences.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of the study. The material preparation, data collection, and analysis were performed by Takashi Funaki, Ryosuke Higuchi, and Mike Saji. The first draft of the manuscript was written by Takashi Funaki, and all the authors commented on the previous versions. All authors have read and approved the final manuscript. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Isobe received honoraria from Medtronic Japan Co., Ltd. (Tokyo, Japan), Abbott Medical Japan LLC (Tokyo, Japan), Boston Scientific Japan K.K. (Tokyo, Japan), BIOTRONIK Japan, Inc. (Tokyo, Japan), Japan Lifeline Co., Ltd. (Tokyo, Japan), TERUMO CORPORATION (Tokyo, Japan), NIPRO CORPORATION (Osaka, Japan), DVx Inc. (Tokyo, Japan), Active Medical Co., Ltd. (Hokkaido, Japan), TORAY INDUSTRIES, INC. (Tokyo, Japan), KANEKA MEDIX CORPORATION (Osaka, Japan), Johnson & Johnson K. K. Medical Company (Tokyo, Japan), Nippon Boehringer Ingelheim Co., Ltd (Tokyo, Japan). Dr. Saji has received consulting fees from Abbott Medical Japan LLC (Tokyo, Japan) and Medtronic Japan Co., Ltd. (Tokyo, Japan). Dr. Takamisawa and Dr. Tobaru are on-site proctors of Medtronic, Inc. (Minneapolis, MN, USA) and Edwards Life Sciences Corporation (Irvine, CA, USA). Dr. Doi is an on-site proctor of Medtronic, Inc. (Minneapolis, MN, USA). Dr. Higuchi is a clinical proctor of Edwards Life Sciences Corporation (Irvine, CA, USA). Takashi Funaki, Mamoru Nanasato, Harutoshi Tamura, Kei Sato, Hiroaki Yokoyama, Shinya Okazaki, Takayuki Onishi, Shuichiro Takanashi, Takanori Ikeda, and Hiroaki Kitaoka declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Funaki, T., Saji, M., Higuchi, R. et al. Impact of osteoporotic risk in men undergoing transcatheter aortic valve replacement: a report from the LAPLACE-TAVI registry. Cardiovasc Interv and Ther (2024). https://doi.org/10.1007/s12928-024-01011-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12928-024-01011-7