Abstract
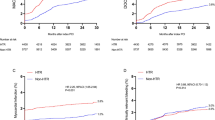
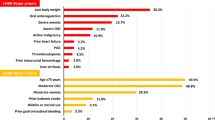
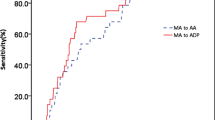
Although the Japanese high bleeding risk criteria (J-HBR) were established to predict bleeding risk in patients undergoing percutaneous coronary intervention (PCI), the thrombogenicity in the J-HBR status remains unknown. Here, we examined the relationships among J-HBR status, thrombogenicity and bleeding events. This study was a retrospective analysis of 300 consecutive patients who underwent PCI. Blood samples obtained on the day of PCI were used in the total thrombus-formation analysis system (T-TAS) to investigate the thrombus-formation area under the curve (AUC; PL18-AUC10 for platelet chip; AR10-AUC30 for atheroma chip). The J-HBR score was calculated by adding 1 point for any major criterion and 0.5 point for any minor criterion. We assigned patients to three groups based on J-HBR status: a J-HBR-negative group (n = 80), a low score J-HBR-positive group (positive/low, n = 109), and a high score J-HBR-positive group (positive/high, n = 111). The primary end point was the 1-year incidence of bleeding events defined by the Bleeding Academic Research Consortium types 2, 3, or 5. Both PL18-AUC10 and AR10-AUC30 levels were lower in the J-HBR-positive/high group than the negative group. Kaplan–Meier analysis showed worse 1-year bleeding event-free survival in the J-HBR-positive/high group compared with the negative group. In addition, both T-TAS levels in J-HBR positivity were lower in those with bleeding events than in those without bleeding events. In multivariate Cox regression analyses, the J-HBR-positive/high status was significantly associated with 1-year bleeding events. In conclusion, the J-HBR-positive/high status could reflect low thrombogenicity as measured by T-TAS and high bleeding risk in patients undergoing PCI.




Similar content being viewed by others
Data availability
The deidentified participant data will not be shared.
References
Palmerini T, Benedetto U, Bacchi-Reggiani L, Della Riva D, Biondi-Zoccai G, Feres F, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385:2371–82.
Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the academic research consortium for high bleeding risk. Eur Heart J. 2019;40:2632–53.
Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140:240–61.
Ueki Y, Bär S, Losdat S, Otsuka T, Zanchin C, Zanchin T, et al. Validation of the academic research consortium for high bleeding risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention. 2020;16:371–9.
Natsuaki M, Morimoto T, Shiomi H, Yamaji K, Watanabe H, Shizuta S, et al. Application of the academic research consortium high bleeding risk criteria in an all-comers registry of percutaneous coronary intervention. Circ Cardiovasc Interv. 2019;12: e008307.
Nakamura M, Kadota K, Nakao K, Nakagawa Y, Shite J, Yokoi H, et al. High bleeding risk and clinical outcomes in East Asian patients undergoing percutaneous coronary intervention: the PENDULUM registry. EuroIntervention. 2021;16:1154–62.
Cao D, Mehran R, Dangas G, Baber U, Sartori S, Chandiramani R, et al. Validation of the academic research consortium high bleeding risk definition in contemporary PCI patients. J Am Coll Cardiol. 2020;75:2711–22.
Nakamura M, Iijima R. Implications and characteristics of high bleeding risk in East Asian patients undergoing percutaneous coronary intervention: start with what is right rather than what is acceptable. J Cardiol. 2021;78:91–8.
Natsuaki M, Morimoto T, Yamaji K, Watanabe H, Yoshikawa Y, Shiomi H, et al. Prediction of thrombotic and bleeding events after percutaneous coronary intervention: CREDO-Kyoto thrombotic and bleeding risk scores. J Am Heart Assoc. 2018;7:e008708.
Nakamura M, Kitazono T, Kozuma K, Sekine T, Nakamura S, Shiosakai K, et al. Prasugrel for Japanese patients with ischemic heart disease in long-term clinical practice (PRASFIT-Practice II) - 1-year follow-up results of a postmarketing observational study. Circ J. 2019;84:101–8.
Numasawa Y, Kohsaka S, Ueda I, Miyata H, Sawano M, Kawamura A, et al. Incidence and predictors of bleeding complications after percutaneous coronary intervention. J Cardiol. 2017;69:272–9.
Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, et al. JCS 2020 guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ J. 2020;84:831–65.
Natsuaki M, Morimoto T, Shiomi H, Ehara N, Taniguchi R, Tamura T, et al. Application of the modified high bleeding risk criteria for Japanese patients in an all-comers registry of percutaneous coronary intervention - from the CREDO-Kyoto registry cohort-3. Circ J. 2021;85:769–81.
Shimizu T, Sakuma Y, Kurosawa Y, Muto Y, Sato A, et al. Validation of Japanese bleeding risk criteria in patients after percutaneous coronary intervention and comparison with contemporary bleeding risk criteria. Circ Rep. 2022. https://doi.org/10.1253/circrep.CR-22-0023.
Kaikita K, Hosokawa K, Dahlen JR, Tsujita K. Total thrombus-formation analysis system (T-TAS): clinical application of quantitative analysis of thrombus formation in cardiovascular disease. Thromb Haemost. 2019;119:1554–62.
Ito M, Kaikita K, Sueta D, Ishii M, Oimatsu Y, Arima Y, et al. Total thrombus-formation analysis system (T-TAS) can predict periprocedural bleeding events in patients undergoing catheter ablation for atrial fibrillation. J Am Heart Assoc. 2016;5:e002744.
Oimatsu Y, Kaikita K, Ishii M, Mitsuse T, Ito M, Arima Y, et al. Total thrombus-formation analysis system predicts periprocedural bleeding events in patients with coronary artery disease undergoing percutaneous coronary intervention. J Am Heart Assoc. 2017;6:e005263.
Mitsuse T, Kaikita K, Ishii M, Oimatsu Y, Nakanishi N, Ito M, et al. Total thrombus-formation analysis system can predict 1-year bleeding events in patients with coronary artery disease. J Atheroscler Thromb. 2020;27:215–25.
Nakanishi N, Kaikita K, Ishii M, Kuyama N, Tabata N, Ito M, et al. Development and assessment of total thrombus-formation analysis system-based bleeding risk model in patients undergoing percutaneous coronary intervention. Int J Cardiol. 2021;325:121–6.
Nakanishi N, Kaikita K, Ishii M, Kuyama N, Tabata N, Ito M, et al. Hemodialysis-related low thrombogenicity measured by total thrombus-formation analysis system in patients undergoing percutaneous coronary intervention. Thromb Res. 2021;200:141–8.
Nakanishi N, Kaikita K, Ishii M, Kuyama N, Tabata N, Ito M, et al. Malnutrition-associated high bleeding risk with low thrombogenicity in patients undergoing percutaneous coronary intervention. Nutr Metab Cardiovasc Dis. 2022;32:1227–35.
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7.
Hill SA, Booth RA, Santaguida PL, Don-Wauchope A, Brown JA, Oremus M, et al. Use of BNP and NT-proBNP for the diagnosis of heart failure in the emergency department: a systematic review of the evidence. Heart Fail Rev. 2014;19:421–38.
Hosokawa K, Ohnishi T, Kondo T, Fukasawa M, Koide T, Maruyama I, et al. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost. 2011;9:2029–37.
Hosokawa K, Ohnishi T, Sameshima H, Miura N, Ito T, Koide T, et al. Analysing responses to aspirin and clopidogrel by measuring platelet thrombus formation under arterial flow conditions. Thromb Haemost. 2013;109:102–11.
Hosokawa K, Ohnishi T, Fukasawa M, Kondo T, Sameshima H, Koide T, et al. A microchip flow-chamber system for quantitative assessment of the platelet thrombus formation process. Microvasc Res. 2012;83:154–61.
Sueta D, Kaikita K, Okamoto N, Arima Y, Ishii M, Ito M, et al. A novel quantitative assessment of whole blood thrombogenicity in patients treated with a non-vitamin K oral anticoagulant. Int J Cardiol. 2015;197:98–100.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–47.
Valle JA, Shetterly S, Maddox TM, Ho PM, Bradley SM, Sandhu A, et al. Postdischarge bleeding after percutaneous coronary intervention and subsequent mortality and myocardial infarction: insights from the HMO research network-stent registry. Circ Cardiovasc Interv. 2016;9:e003519.
Palmerini T, Bacchi Reggiani L, Della Riva D, Romanello M, Feres F, Abizaid A, et al. Bleeding-related deaths in relation to the duration of dual-antiplatelet therapy after coronary stenting. J Am Coll Cardiol. 2017;69:2011–22.
Kaikita K, Yasuda S, Akao M, Ako J, Matoba T, Nakamura M, et al. Bleeding and subsequent cardiovascular events and death in atrial fibrillation with stable coronary artery disease: insights from the AFIRE trial. Circ Cardiovasc Interv. 2021;14: e010476.
Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103–13.
Palmerini T, Stone GW. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: conceptual evolution based on emerging evidence. Eur Heart J. 2016;37:353–64.
Malinin A, Pokov A, Spergling M, Defranco A, Schwartz K, Schwartz D, et al. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12(R) rapid analyzer: the VERIfy thrombosis risk assessment (VERITAS) study. Thromb Res. 2007;119:277–84.
Zafar MU, Santos-Gallego CG, Badimon L, Badimon JJ. Badimon perfusion chamber: an ex vivo model of thrombosis. Methods Mol Biol. 2018;1816:161–71.
Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br J Haematol. 2016;174:503–14.
Tóth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost. 2006;96:781–8.
Favaloro EJ. Clinical utility of the PFA-100. Semin Thromb Hemost. 2008;34:709–33.
Sato Y, Yoshihisa A, Takeishi R, Ohara H, Sugawara Y, Ichijo Y, et al. B-type natriuretic peptide is associated with the occurrence of bleeding events in heart failure patients with a history of coronary artery disease. J Cardiol. 2022;80:88–93.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147-239.
Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure - digest version. Circ J. 2019;83:2084–184.
Acknowledgements
We thank K. Hosokawa and T. Ohnishi from the Research Institute, Fujimori Kogyo Co., Yokohama, Kanagawa, Japan, for their excellent technical support in operating the T-TAS®. We also thank all paramedical staff members and clinical secretaries for their kind support during this work.
Funding
This study was supported in part by grants-in-aid for Scientific Research (#15K09089 and #18K08110) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Koichi Kaikita has received remuneration for lecturing from Bayer Yakuhin, Ltd., Daiichi-Sankyo Co., Ltd., Novartis Pharma AG., and Otsuka Pharmaceutical Co., Ltd.; trust research/joint research funds from SBI Pharmaceuticals Co., Ltd., Bayer Yakuhin, Ltd., and Daiichi-Sankyo Co., Ltd.; and scholarship funds from Abbott Medical Co., Ltd. Dr. Kenichi Tsujita has received remuneration for lecturing from Amgen Astellas BioPharma K.K., Bayer Yakuhin, Ltd., Bristol-Myers K.K., Daiichi Sankyo Co., Ltd., Kowa Pharmaceutical Co. Ltd., MSD K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., ITI Co., Ltd., trust research/joint research funds from Bristol-Myers K.K., Kowa Pharmaceutical Co. Ltd., and scholarship funds from Abbott Medical Japan L.L.C, Abbott Vascular Japan Co., Ltd., Boston Scientific Japan K.K., Cardinal Health Japan, Chugai Pharmaceutical Co, Ltd., Fides-one, Inc., Fukuda Denshi Co., Ltd., Japan Lifeline Co., Ltd., Kaneka Medix Co., Ltd., Medtronic Japan Co., Ltd., Mitsubishi Tanabe Pharma, NIHON KOHDEN CORPORATION, NIPRO CORPORATION, Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and TERUMO Co, Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakanishi, N., Kaikita, K., Ishii, M. et al. Japanese high bleeding risk criteria status predicts low thrombogenicity and bleeding events in patients undergoing percutaneous coronary intervention. Cardiovasc Interv and Ther 38, 299–308 (2023). https://doi.org/10.1007/s12928-023-00920-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-023-00920-3