Abstract
Coronary artery disease (CAD) and severe aortic valve stenosis frequently coexist. Given the progressive nature of CAD, silent or non-significant CAD may become symptomatic or functionally relevant years after TAVR. However, there is a paucity of data documenting the feasibility of either coronary angiography and/or PCI after TAVR. We systematically searched Medline, Pubmed, Embase, Cochrane database, Google Scholar, Science Direct, Web of Science, and conference abstracts from conception to March 2020 using OvidSP in TAVR patients undergoing coronary angiography with or without PCI at least 6 months after TAVR. Patients and procedural characteristics were summarized. The primary outcome of interest was successful coronary angiography for either the left main coronary artery (LMCA) or right coronary artery (RCA) with or without PCI. Pooled estimates were calculated using a random-effects meta-analysis. The study protocol was registered in PROSPERO. Eleven reports for a total of 696 coronary angiograms and 287 PCI were included in the analysis. Patients were slightly predominantly male, older and had a mean left ventricular ejection fraction of more than 50% with an intermediate STS. The summary estimate rates of successful LMCA and RCA angiography with a Medtronic self-expandable valve (SEV) were 84% (95% CI 73–90%, I2 = 79, p = 0.015) and 69% (95% CI 37–89%, I2 = 86, p = 0.23), respectively, while with the Edwards Lifesciences balloon expandable valve (BEV), the summary estimate rates for successful LMCA and RCA angiography were 94% (95% CI 72–99%, I2 = 66, p = 0.003) and 95% (95% CI 48–99%, I2 = 83, p = 0.05), respectively. The summary estimate rate of successful PCI post TAVR with either a Medtronic SEV or Edwards Lifesciences BEV was 93% (95% CI 86–96%, I2 = 33, p = 0.0001). The overall achievement of a successful coronary angiography with or without PCI in post-TAVR patients is high, with a lower success rate for RCA angiography in patients with the Medtronic SEV Mortality and bleeding did not differ in our analysis.
Similar content being viewed by others
Introduction
Based on the positive results of the PARTNER 3 [1] and the Evolut Low Risk [2] trials, the FDA has approved, in 2019, transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis in low-risk patients. Coronary artery disease (CAD) and severe aortic valve stenosis frequently coexist. CAD is prevalent in > 60% of patients undergoing surgical aortic valve replacement (SAVR) [3] and up to 65% of patients undergoing TAVR [4]. Given the progressive nature of CAD, silent or non-significant CAD may become symptomatic or functionally relevant years after TAVR. Consequently, there will be an increasing number of patients with TAVR valves presenting with progressive CAD or acute coronary syndrome (ACS) in the coming years [5].
Recently, the Center of Medicare and Medicaid Services (CMS), supported by the American College of Cardiology (ACC), has expanded the coverage of percutaneous coronary interventions (PCI) at ambulatory centers in the United States of America (USA) [6], so it is expected more of coronary angiography with or without PCI to be done in non-TAVR centers or even outpatient settings with limited exposure to transcatheter valve interventions.
However, there is a paucity of data documenting the feasibility of either delayed coronary angiography and/or PCI after TAVR. Success rates have varied with challenges reported, particularly with the self-expanding supra-annular valve [7, 8]. It is therefore essential for the diagnostic and interventional cardiologists to understand the potential challenges of coronary angiography and PCI in this patient population being particularly useful in time-critical scenarios, such as ACS [9].
We, therefore, undertook a systematic literature review and meta-analysis of studies examining the feasibility of delayed coronary angiography with or without PCI and its clinical outcomes in patients who underwent TAVR.
Methods
Search strategy
We conducted a search of Medline, Pubmed, Embase, Cochrane database, Google Scholar, Science Direct, Web of Science, and conference abstracts from conception to March 2020 using OvidSP. The following terms were used: (transcatheter aortic valve implantation OR transfemoral aortic valve implantation OR transapical aortic valve implantation OR trans-subclavian aortic valve implantation OR TAVI OR transcatheter aortic valve replacement OR TAVR) AND (coronary angiography OR selective coronary angiography OR coronary artery catheterization OR left heart catheterization) AND/OR (percutaneous coronary intervention OR PCI OR coronary angioplasty OR plain balloon angioplasty or POBA).
Studies published other than the English language were translated into English using Google Translator. This systematic review was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISMA) [10]. We followed an a priori protocol. This study used only aggregate de-identified data and was therefore exempt from additional review board review.
Study selection
The abstract and titles yielded by the search were screened by two independent investigators (A.R. and F.M.) against the inclusion criteria. Three other investigators (P.V. and J.V.) carefully analyzed the exclusion criteria and secondary outcomes whenever there was a potential article to be included in this study. Additional studies were retrieved by checking the bibliography of included studies and relevant reviews. The full reports of potentially relevant studies were retrieved, and data were independently extracted on study design, participant characteristics, treatment groups, outcome events, follow-up, and results. Any discrepancies between reviewers were resolved by discussion with the two leading study investigators (M.A.D and M.P.). The authors for the included or excluded studies were not contacted. This study protocol was registered in PROSPERO.
Eligibility and exclusion criteria
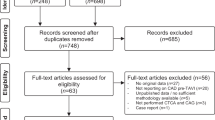
We included only studies that evaluated patients who underwent TAVR at least 6 months before coronary angiography and/or PCI. Most of the TAVR procedural complications happen within the first 30 days after the procedure and after 3 months, there is considerable amount of re-endothelization of and around the bio-prosthesis [11], reason why a 6-month threshold was chosen, to reflect a more precise answer to our research question. Since most of the commercial valves available for TAVR are either the Medtronic SEV or Edwards Lifescience BEV, only these were included in our analysis. In terms of outcomes, studies included must have evaluated the rate of successful coronary artery cannulation for both left main coronary artery (LMCA) and right coronary artery (RCA), and secondary outcomes when reported by an individual study whenever available: death, procedure-related bleeding, procedure time, radiation dose, and contrast volume. We excluded isolated case reports or case series (≤ 3 patients), reviews, studies with the inappropriate study design, TAVR related complications, such as coronary thrombosis, due to leaflet failure or coronary obstruction, coronary procedures done before or at the same TAVR index hospitalization, coronary angiography and/or PCI to vein grafts, and editorial comments on the subjects. When duplicate reports of the same study were identified, only the report with the most complete data set and detailed methodology description was included. Since the definition of successful coronary angiography varies among studies, we considered successful, the definition of each study has used for its quantitative analysis. A flow diagram is provided following PRISMA (Fig. 1).
Quality and risk of bias assessment
To assess the quality of included cohort studies, we used the Newcastle–Ottawa Scale (NOS) [12]. Findings were resumed in Table 3. The outcomes of interest and follow-up were also extracted on a preformatted table. Disagreements were resolved by consensus after consultation with two investigators (M.A.D. and M.P.). The risk of bias was assessed by considering the ascertainment of treatment groups, the ascertainment of outcomes, loss to follow-up, and potential confounders in the data analysis.
Data analysis
We calculated the event rate of the primary and secondary outcomes of interest from each study. Each successful cannulation counted as a successful event rate (average of 2 per patient; one count for the LMCA and RCA). We then pooled the log-transformed event rates using the DerSimonian and Laird random-effects models with the heterogeneity estimated from the Mantel–Haenszel model. All analyses were performed using the random-effect model to produce relative risks and 95% confidence intervals (CIs). Heterogeneity was assessed using the I2 statistic. This statistic represents the proportion of variability that is not attributable to chance, with I2 < 25% considered low, I2 values of 25–50% considered moderate, and I2 > 75% considered high statistical heterogeneity. If there were insufficient data or studies for meta-analysis, we pooled the studies using a weighted average or performed narrative synthesis of studies that were too heterogeneous to pool. Statistical analyses were conducted using Open Meta [Analyst] [13] and Stata 16 (College Station, TX, USA). All values were two-tailed, and a p ≤ 0.05 was set as the threshold for statistical significance [14, 15].
Results
Search results
Our search retrieved a total of 447 entries, which were reduced to 159 studies after an initial pre-screening. Eighty-two studies were excluded based on the excluded criteria. In the assessment of eligibility, two additional studies were excluded because TAVR was performed in less than 6 months prior to coronary angiography or PCI [5, 8] but were included in subsequent secondary analysis (data not published). Finally, a total of 11 studies [7, 16,17,18,19,20,21,22,23,24,25] with a median follow-up of at least 6 months were available for the analysis. The study selection procedure is reported in detail in Fig. 1.
Study characteristics
Table 1 summarizes each study design, the country of origin, and the reason each study was included in this analysis with the definition of successful coronary angiography or PCI. Table 2 summarizes the patient's most relevant baseline characteristics for each study. Across studies, patients were slightly predominantly male, older and had a mean left ventricular ejection fraction of more than 50% with an intermediate STS. The absolute number and percentages of patients with the previous CAD, previous MI, and PCI were also available (Table 2).
Quality assessment
All the available studies were non-randomized (two were comparative, and nine were non-comparative). Most of the non-comparative showed a NOS average quality in terms of selection of study participants, ascertainment of exposure, control for confounding, and ascertainment of the outcome. The overall judgment of the quality of the study was between average and high. The risk of bias assessment of each included study is summarized in Table 3.
Meta-analysis for the primary outcomes
The analysis of this study primary outcome, including all results for the successful coronary angiography for the LMCA and RCA, is shown in Fig. 2. The summary estimate rate of successful LMCA angiography with a Medtronic SEV was 84% (95% CI 73–90%, I2 = 79, p = 0.015) while with the Edwards Lifescience BEV, the summary estimate rate was 94% (95% CI 72–99%, I2 = 66, p = 0.003). (Panel a for the Medtronic SEV and panel b for the Edwards Lifescience BEV). The summary estimate rate of successful RCA angiography with a Medtronic SEV was 69% (95% CI 37–89%, I2 = 86, p = 0.23) while with the Edwards Lifescience BEV valve the summary estimate rate was 95% (95% CI 48–99%, I2 = 83, p = 0.05). (Panel c for the Medtronic SEV and panel d for the Edwards Lifescience BEV). The primary analysis on the composite endpoint of successful PCI for both Medtronic SEV and Edwards Lifescience BEV, including all results of the studies is presented in Fig. 2—panel e. The summary estimate rate of successful PCI post TAVR with either a Medtronic SEV or Edwards Lifescience BEV was 93% (95% CI 86–96%, I2 = 33, p = 0.0001). Supplemental analysis with the correspondent funnel plot for each meta-analysis was also carried out (supplementary figure).
Random effects meta-analysis of successful LMCA angiography in patients with a Medtronic SEV (panel a); and in patients with an Edwards Lifescience BEV (panel b). Random effects meta-analysis of successful RCA angiography in patients with a Medtronic SEV (panel c); and in patients with an Edwards Lifescience BEV (panel d). Panel e random-effects meta-analysis of successful PCI in post-TAVR patients, performed at least 6 months after TAVR
Meta-analysis for the secondary outcomes
The secondary endpoints are reported in Fig. 3 for mortality and bleeding. The summary estimate rate of death was 0.7% (95% CI 0.3–1.7%, p = 0.88) and for bleeding, the summary estimate was 0.5% (95% CI 0.2–0.65%, p = 0.91). For the studies which compared procedural PCI characteristics pre-TAVR versus matched post-TAVR patients, further analysis was performed on the mean and S.D, as shown in Fig. 4. A two-way ANOVA for procedure time, fluoroscopy time and contrast volume were, respectively, 58.18 ± 39.96 min, 18.25 ± 21.26 min and 156.95 ± 87.2 ml in pre-TAVR patients versus 56.87 ± 26.59 min, 19.25 ± 11.77 min and 147 ± 61.6 ml in post-TAVR patients (p > 0.05).
Discussion
With the advancement in structural cardiology, TAVR has become the standard treatment option for patients with severe calcific aortic stenosis who are considered intermediate or high surgical risks, and more recently, FDA has expanded this therapy to low-risk patients. It has been shown that the prevalence of CAD in TAVR ranged from 55 to 70% [26, 27].
Despite a high prevalence of CAD in TAVR patients, since both conditions overlap on its pathophysiology, there is no clear consensus when to treat severe CAD, either pre- or post-TAVR. Griese et al. showed a statistically higher 30-day cardiovascular mortality in patients who received PCI (either staged before TAVR or combined with TAVR) compared to those who underwent isolated TAVR [28]. In contrast, Pasic et al. showed favorable 1-year survival in patients who underwent PCI and transapical TAVR in the same session [29]. Recently, and despite several limitations, Kotronias et al. have showed in a detailed and comprehensive meta-analysis that revascularization before or during TAVR confers no clinical advantage with respect to several patient-important clinical outcomes and may be associated with an increased risk of major vascular complications and 30-day mortality [30]. Despite the lack of consensus and until the results of the ACTIVATION (The Percutaneous Coronary Intervention Prior to Transcatheter Aortic Valve Implantation) trial that aims to test the hypothesis that revascularization of significant coronary artery disease by PCI prior to TAVR, pre-TAVR PCI is a practice widely accepted in the interventional cardiologist community.
TAVR indications continue to expand to lower risk younger and less morbid patients, who also exhibit longer life expectancy as shown in two randomized control trials and its indication might continue to grow depending on the results of the highly anticipated EARLY TAVR (Evaluation of Transcatheter Aortic Valve Replacement Compared to SurveilLance for Patients With AsYmptomatic Severe Aortic Stenosis; NCT03042104) trial that compares TAVR to routine surveillance in patients with asymptomatic severe AS.
Undoubtedly, there will be an increasing number of patients with TAVR valves presenting with progressive CAD or acute coronary syndrome in the coming years. This will occur at both major tertiary non-TAVR centers and at community hospitals. Moreover, with the current expansion of PCI procedures at the ambulatory in the United States, most of these procedures are anticipated to be done in the outpatient settings with limited exposure to transcatheter valve interventions [9]. It is therefore essential for diagnostic and interventional cardiologists to understand the potential challenges of coronary angiography and PCI in this population.
This meta-analysis tried to address how successful coronary angiography with or without PCI can be achieved after TAVR, using a Medtronic SEV or Edwards Lifescience BEV since these two represent over 90% of TAVR devices implanted worldwide. For this, we excluded coronary access or PCI related to the TAVR procedure itself or done during the same TAVR index hospitalization and included only studies where the delayed coronary angiography with or without PCI was performed at least 6 months after TAVR. As far as the authors are aware, this is the first meta-analysis of its kind published in the English literature.
The results of this meta-analysis of 11 retrospective studies included 696 coronary angiograms. Despite the definition of successful coronary angiography being highly heterogeneous across studies, the overall rate of a successful delayed coronary access is highly achievable. Considering the type of valve, the estimated rate of successful LMCA angiography with a Medtronic SEV was 84% (p = 0.015) while with the valve, this rate was 94% (p = 0.003). On the other hand, the estimated rate of successful RCA angiography with a Medtronic SEV was only 69% (p = 0.23) while with the Edwards Lifescience BEV the summary estimate rate rose to 95% (p = 0.05). This could be explained partially by the fact that when deploying a SEV, given its design, extra attention should be paid to the position of the skirt and commissural posts relative to the coronary ostia and that engaging from a diamond below the ostia has been associated with cannulation difficulties [9, 31, 32]. Based on recent research findings, it seems the low success rate of RCA cannulation post-Medtronic SEV also could be explained by both clinical and anatomical features [8, 22]. Barbanti et al. recently showed in the RE-ACCESS study that unsuccessful coronary cannulation following TAVR was observed in 7.7% of patients and occurred almost exclusively in those receiving the Medtronic SEV. The combination of Evolut TAV, a higher TAV–sinus of Valsalva relation, and implantation depth predicts with high accuracy the risk for unsuccessful coronary cannulation after TAVR [33].
In our study, we found delayed 287 PCI were performed post TAVR with an overall success rate of 93% with either a Medtronic SEV or Edwards Lifescience BEV (95% CI 86–96%, I2 = 33, p = 0.0001). The mortality and bleeding rates are low in this cohort, around 0.7% without a statistical significance (p = 0.88 and p = 0.91, respectively). There was no statistical difference regarding the procedure time, fluoroscopy time and contrast volume in post-TAVR patients undergoing PCI at least 6 months after. These were, respectively, 58.18 ± 39.96 min, 18.25 ± 21.26 min, and 156.95 ± 87.2 ml in pre-TAVR patients versus 56.87 ± 26.59 min, 19.25 ± 11.77 min, and 147 ± 61.6 ml (p > 0.05) in matched post-TAVR patients. Our findings were also in concordance with previous studies not included in this meta-analysis [5, 8]. At a first glance, the lack of difference can be of a concern, and a plausible explanation could be illustrated by the fact that in post-TAVR patients a lot is already known in regards their coronary anatomy, best arterial access, and degree of coronary disease and potential risks for worsening renal function. All these factors together and possible foreseen complications in regards coronary re-access could substantially decrease radiation and contrast volume [32].
Conclusion
In conclusion, this is the first systematic review and meta-analysis that showed that delayed coronary angiography and PCI in patients after TAVR is highly achievable with a low mortality and bleeding rates. Intricate knowledge of the valve design and its relationship with the coronary ostia could be especially important in attempting coronary cannulation, especially when trying to cannulate the RCA in a previously implanted self-expanding valve. The data presented in this study can be reassuring since more of the procedures will be performed in non-TAVR centers.
Limitations
This study has several limitations mainly the data retrieved are based on observational studies, with limited follow-up, performed in TAVR centers by experienced TAVR and seasoned coronary interventional operators, and therefore may not be applicable to lower risk cohorts with greater life expectancy done in non-TAVR centers by operators with limited exposure to transcatheter aortic valve therapies. Randomized controlled trials are very needed to determine the role of coronary angiography with or without PCI in patients with TAVR in low-risk patients. Meanwhile, in the absence of definitive evidence, careful evaluation of patients on an individual’s basis is of paramount importance to determine if these procedures can be safely carried out in the outpatient and non-TAVR centers, and for whom the benefits are carefully balanced against the potential catastrophic risks.
References
Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–705.
Popma J, Deeb M, Yakubov S, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–15.
Kvidal P, Bergström R, Hörte LG, Ståhle E. Observed and relative survival after aortic valve replacement. J Am CollCardiol. 2000;35(3):747–56.
D’Ascenzo F, Conrotto F, Giordana F, et al. Mid-term prognostic value of coronary artery disease in patients undergoing transcatheter aortic valve implantation: a meta-analysis of adjusted observational results. Int J Cardiol. 2013;168(3):2528–32.
Zivelonghi C, Pesarini G, Scarsini R, Lunardi M, Piccoli A, Ferrero V, Gottin L, Vassanelli C, Ribichini F. Coronary catheterization and percutaneous interventions after transcatheter aortic valve implantation. Am J Cardiol. 2017;120(4):625–31. https://doi.org/10.1016/j.amjcard.2016.10.046.
Neale T. CMS finalizes rule allowing reimbursement of PCI in ambulatory centers. 2019. https://oklahoman.com/article/5654349/medicare-now-covers-outpatient-coronary-procedure.
Allali A, El-Mawardy M, Schwarz B, et al. Incidence, feasibility and outcome of percutaneous coronary intervention after transcatheter aortic valve implantation with a self-expanding prosthesis. Results from a single center experience. CardiovascRevasc Med. 2016;17(6):391–8.
Boukantar M, Gallet R, Mouillet G, et al. Coronary procedures after TAVI with the self-expanding aortic bioprosthesismedtronicCoreValve TM, not an easy matter. J IntervCardiol. 2017;30(1):56–62.
Yudi MB, Sharma SK, Tang GHL, Kini A. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am CollCardiol. 2018;71(12):1360–78.
Mother D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
van Kesteren F, Wiegerinck E, Rizzo S, et al. Autopsy after transaortic valve implantation. Virchows Arch. 2017;470:331–9.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 3 Mar 2020.
Wallace B, Dahabreh I, Trikalinos T, et al. Closing the gap between methodologists and end users: R as a computational back-end. J Stat Softw. 2012;49:1–15.
Higgins J. Thompson, Simon, Deeks, Jonathan, Altman, Douglas. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Blumenstein J, Kim WK, Liebetrau C, et al. Challenges of coronary angiography and intervention in patients previously treated by TAVI. Clin Res Cardiol. 2015;104(8):632–9.
Katsanos S, Debonnaire P, van der Kley F, et al. Position of Edwards SAPIEN transcatheter valve in the aortic root in relation with the coronary ostia: implications for percutaneous coronary interventions. Catheter CardiovascInterv. 2015;85(3):480–7.
Chakravarty T, Sharma R, Abramowitz Y, et al. Outcomes in patients with transcatheter aortic valve replacement and left main stenting: the TAVR-LM registry. J Am CollCardiol. 2016;67(8):951–60.
Chetcuti S, Kleiman NS, Matthews R, Popma J, Moore J. TCT-743 percutaneous coronary intervention after self-expanding transcatheter aortic valve replacement. J Am CollCardiol. 2016;68:B300–1.
Htun WW, Grines C, Schreiber T. Feasibility of coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement using a Medtronic™ self-expandable bioprosthetic valve. Catheter Cardiovasc Interv. 2018;91(7):1339–44. https://doi.org/10.1002/ccd.27346.
Ferreira-Neto AN, Puri R, Asmarats L, et al. Clinical and technical characteristics of coronary angiography and percutaneous coronary interventions performed before and after transcatheter aortic valve replacement with a balloon-expandable valve. J IntervCardiol. 2019;2019:3579671.
Vilalta V, Asmarats L, Ferreira-Neto AN, et al. Incidence, clinical characteristics, and impact of acute coronary syndrome following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11(24):2523–33.
Faroux L, Munoz-Garcia E, et al. Acute coronary syndrome following transcatheter aortic valve replacement. CircCardiovascInterv. 2020;13(2):e008620.
Couture T, Faroux L, Junquera L, et al. Interaction between self-expanding transcatheter heart valves and coronary ostia: an angiographically based analysis of the Evolut R/Pro valve system. J Invasive Cardiol. 2020. Cited in: Ovid MEDLINE(R) Epub Ahead of Print. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medp&NEWS=N&AN=32160151. Accessed 31 Mar 2020.
Tanaka A, Jabbour RJ, Testa L, et al. Incidence, technical safety, and feasibility of coronary angiography and intervention following self-expanding transcatheter aortic valve replacement. CardiovascRevasc Med. 2019;20(5):371–5.
Wenaweser P, Pilgrim T, Guerios E, et al. Impact of coronary artery disease and percutaneous coronary intervention on outcomes in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention. 2011;7(5):541–8.
Gautier M, Pepin M, Himbert D, et al. Impact of coronary artery disease on indications for transcatheter aortic valve implantation and on procedural outcomes. EuroIntervention. 2011;7(5):549–55.
Griese DP, Reents W, Tóth A, et al. Concomitant coronary intervention is associated with poorer early and late clinical outcomes in selected elderly patients receiving transcatheter aortic valve implantation. Eur J CardiothoracSurg. 2014;46(1):e1–7.
Pasic M, Dreysse S, Unbehaun A, et al. Combined elective percutaneous coronary intervention and transapicaltranscatheter aortic valve implantation. Interact CardiovascThoracSurg. 2012;14(4):463–8.
Kotronias RA, Kwok CS, George S, et al. Transcatheter aortic valve implantation with or without percutaneous coronary artery revascularization strategy: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(6):1–27.
Harhash A, Ansari J, Mandel L, Kipperman R. STEMI after TAVR: procedural challenge and catastrophic outcome. JACC CardiovascInterv. 2016;9(13):1412–3.
Chodor P, Wilczek K, Przybylski R, Nozynski J, Wloch L, Kalarus Z. Percutaneous access to coronary arteries in patients after transcatheter aortic valve implantation procedures - is it a real problem? PostepyKardiolInterwencyjnej. 2019;15(3):274–82.
Barbanti M, Costa G, Picci A, et al. Coronary cannulation after transcatheter aortic valve replacement: the RE-ACCESS study. JACC CardiovascInterv. 2020;13(21):2542–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial conflicts to disclose. All authors contributed equally to this manuscript. This study used only aggregate de-identified data and was therefore exempt from additional review board review and previous informed consent, strictly following the Declaration of Helsinki and the NIH guidelines involving human subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diaz, M.A., Patton, M., Valdes, P. et al. A systematic review and meta-analysis of delayed coronary artery access for coronary angiography with or without percutaneous coronary intervention (PCI) in patients who underwent transcatheter aortic valve replacement (TAVR). Cardiovasc Interv and Ther 37, 167–181 (2022). https://doi.org/10.1007/s12928-020-00753-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-020-00753-4