Abstract
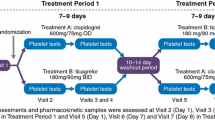
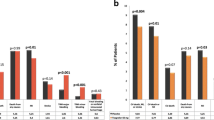
This randomized, active-controlled, double-blind study assessed the pharmacodynamics, pharmacokinetics and safety of ticagrelor in Japanese patients and a smaller cohort of non-Japanese Asian patients. The study recruited patients aged 20–80 years who had received aspirin 75–100 mg/day for ≥2 weeks and had percutaneous coronary intervention or acute coronary syndrome >3 months previously. Patients received 4 weeks’ treatment with ticagrelor 45 mg bid, ticagrelor 90 mg bid or clopidogrel 75 mg qd (all with aspirin). The inhibition of platelet aggregation (IPA, final-extent) and pharmacokinetics of ticagrelor were assessed on days 1 and 28. Overall, 139 Asian patients were randomized (ticagrelor 45 mg bid, n = 50; ticagrelor 90 mg bid, n = 43; clopidogrel, n = 46) of whom 118 were Japanese. Mean final-extent IPA was greater with ticagrelor 90 mg bid versus ticagrelor 45 mg bid and with both ticagrelor doses versus clopidogrel. At the end of the dosing interval on day 28, mean final-extent IPA was 10.0 % higher (95 % confidence interval 0.5–19.5 %) for ticagrelor 90 mg bid versus ticagrelor 45 mg bid, 15.1 % higher (5.8–24.4 %) for ticagrelor 45 mg bid versus clopidogrel, and 25.1 % higher (15.5–34.7 %) for ticagrelor 90 mg bid versus clopidogrel. In Japanese patients, exposure to ticagrelor and its active metabolite AR-C124910XX increased dose-proportionally. The safety profile of ticagrelor was consistent with previous studies. Ticagrelor was associated with enhanced IPA versus clopidogrel in Japanese patients.



Similar content being viewed by others
References
Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038–47.
van Giezen JJ, Nilsson L, Berntsson P, Wissing BM, Giordanetto F, Tomlinson W, et al. Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemostas. 2009;7:1556–65.
Husted S. Evaluating the risk-benefit profile of the direct-acting P2Y(12) inhibitor ticagrelor in acute coronary syndromes. Postgrad Med. 2011;123:79–90.
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57.
Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol. 2010;70:65–77.
Teng R, Butler K. AZD6140, the first reversible oral platelet P2Y12 receptor antagonist, has linear pharmacokinetics and provides near complete inhibition of platelet aggregation, with reversibility of effect in healthy subjects. Can J Clin Pharmacol. 2010;15:e426.
Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y(12) receptor antagonist, in healthy subjects. Eur J Clin Pharmacol. 2010;66:487–96.
Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–21.
Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug–drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction and differential kinetics. Drug Metab Dispos. 2011;39:703–10.
Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–85.
Hasan MS, Basri HB, Hin LP, Stanslas J. Genetic polymorphisms and drug interactions leading to clopidogrel resistance: why the Asian population requires special attention. Int J Neurosci. 2013;123:143–54.
Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75.
Roden DM, Stein CM. Clopidogrel and the concept of high-risk pharmacokinetics. Circulation. 2009;119:2127–30.
Kimura T, Morimoto T, Nakagawa Y, Kawai K, Miyazaki S, Muramatsu T, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation. 2012;125:584–91.
Teng R, Butler K. Comparison of the pharmacokinetics, pharmacodynamics and tolerability of single and multiple doses of ticagrelor in healthy Japanese and Caucasian volunteers. Int J Clin Pharmacol Ther. 2013 [Epub ahead of print].
Labarthe J, Théroux P, Angioï M, Ghitescu M. Matching the evaluation of the clinical efficacy of clopidogrel to platelet function tests relevant to the biological properties of the drug. J Am Coll Cardiol. 2005;46:638–45.
Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2299–306.
James S, Åkerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, et al. Comparison of ticagrelor, the first reversible oral P2Y12 receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet Inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599–605.
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502.
Hoshino K, Horiuchi H, Tada T, Tazaki J, Nishi E, Kawato M, et al. Clopidogrel resistance in Japanese patients scheduled for percutaneous coronary intervention. Circ J. 2009;73:336–42.
Yokoi H, Kimura T, Isshiki T, Ogawa H, Ikeda Y. Pharmacodynamic assessment of a novel P2Y12 receptor antagonist in Japanese patients with coronary artery disease undergoing elective percutaneous coronary intervention. Thromb Res. 2012;129:623–8.
Li H, Butler K, Yang L, Yang Z, Teng R. Pharmacokinetics and tolerability of single- and multiple-doses of ticagrelor in healthy Chinese volunteers. Clin Drug Investig. 2012;32:87–97.
Acknowledgments
Medical writing support was provided by Rick Flemming and David Evans at Gardiner Caldwell Communications and was funded by AstraZeneca. The authors acknowledge Takaaki Isshiki (Teikyo University School of Medicine), Hiroyuki Daida (Juntendo University Faculty of Medicine), Yasuo Ikeda (Waseda University School of Advanced Science and Engineering), Hisao Ogawa (Kumamoto University School of Medicine) and Makoto Takagi (Saiseikai Central Hospital), who were members of the data safety monitoring board. Technical training in the use of platelet aggregation equipment and the surveillance of aggregation curve accuracy was provided to staff from all sites by Laura West of the University of Sheffield. The authors would also like to thank all medical staff and patients involved in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiasa, Y., Teng, R. & Emanuelsson, H. Pharmacodynamics, pharmacokinetics and safety of ticagrelor in Asian patients with stable coronary artery disease. Cardiovasc Interv and Ther 29, 324–333 (2014). https://doi.org/10.1007/s12928-014-0277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-014-0277-1