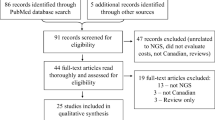
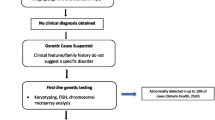
Abstract
High-throughput next-generation sequencing technologies have seen an increase in use in most developed countries. The translation of genomic testing into clinical practice challenges the traditional model of medical care in France and raises numerous medical, legal, ethical, organizational, and financial issues. In order to allow the population to use this revolution to its advantage, France has conceived the French Plan for Genomic Medicine 2025. Its aim is to improve health and quality of life, to organize new pathways of care and counseling, and to make decisions about insurance coverage. It has also been designed to drive innovation and promote economic growth in France by incorporating genomic medicine into the French health care system. These issues can be addressed through evaluations developed to aid the decision-making process in the context of resource scarcity. Health economists can help to resolve these resource allocation challenges by measuring the impact of this technological revolution on patients, caregivers, providers, and the health care system. In this paper, we will review challenges associated with implementing genomic testing in France. One of the pilot studies of the French Plan for Genomic Medicine 2025 will be presented as an illustration of the role of health economists in overcoming some of the challenges of this technological revolution.


Similar content being viewed by others
References
Aviesan France Médecine Génomique (2018) 2025 http://www.defiscience.fr/wp-content/uploads/2016/09/22.06.2016_rapport_france_medecine_genomique_2025.pdf Accessed 29 Oct 2018
Callier SL, Abudu R, Mehlman MJ, Singer ME, Neuhauser D, Caga-Anan C, Wiesner GL (2016) Ethical, legal, and social implications of personalized genomic medicine research: current literature and suggestions for the future. Bioethics 30(9):698–705. https://doi.org/10.1111/bioe.12285
Commission évaluation économique et de santé publique (CEESP) Activity Report (2017) https://www.has-sante.fr/portail/upload/docs/application/pdf/2018-07/rapport_activite_ceesp_2017.pdf Accessed 19 Mar 2019
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL (2005) Methods for the eocnomic evaluation of health care programmes. University Press, Oxford
Foster MW, Mulvihill JJ, Sharp RR (2009) Evaluating the utility of personal genomic information. Genet Med 11(8):570–574. https://doi.org/10.1097/GIM.0b013e3181a2743e
Gainotti S, Mascalzoni D, Bros-Facer V, Petrini C, Floridia G, Roos M, Salvatore M, Taruscio D (2018) Meeting patients’ right to the correct diagnosis: ongoing international initiatives on undiagnosed rare diseases and ethical and social issues. Int J Environ Res Public Health 15(10):1–16. https://doi.org/10.3390/ijerph15102072
Grosse SD, Khoury MJ (2006) What is the clinical utility of genetic testing? Genet Med 8(7):448–450. https://doi.org/10.1097/01.gim.0000227935.26763.c6
HAS (2011) Choix méthodologiques pour l’évaluation économique à la HAS. https://www.has-sante.fr/portail/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf. Accessed 29 Oct 2018
HAS (2018) Projet stratégique 2013–2016. https://www.has-sante.fr/portail/upload/docs/application/pdf/2013-09/projet_strategique_synthese.pdf. Accessed 29 Oct 2018
Inserm (2018) Déficiences intellectuelles. Expertise collective. Synthèse et recommandations. https://www.inserm.fr/information-en-sante/expertises-collectives/deficiences-intellectuelles Accessed 29 Oct 2018
Institute of Medicine; Board on Health Sciences Policy; Roundtable on Translating Genomic-Based Research for Health; Adam C. Berger and Steve Olson (2013) The economics of genomic medicine: workshop summary health. Washington (DC): National Academies Press (US)
Kohler JN, Turbitt E, Biesecker BB (2017) Personal utility in genomic testing: a systematic literature review. Eur J Hum Genet 25(6):662–668. https://doi.org/10.1038/ejhg.2017.10
Lethimonnier F, Levy Y (2018) Genomic medicine France 2025. Ann Oncol 29(4):784–785. https://doi.org/10.1093/annonc/mdy027
Mollison L, O’Daniel JM, Henderson GE, Berg JS, Skinner D (2020) Parents’ perceptions of personal utility of exome sequencing results. Genet Med 22(4):752–757. https://doi.org/10.1038/s41436-019-0730-8
Monroe GR, Frederix GW, Savelberg SM, de Vries TI, Duran KJ, van der Smagt JJ, Terhal PA, van Hasselt PM, Kroes HY, Verhoeven-Duif NM, Nijman IJ, Carbo EC, van Gassen KL, Knoers NV, Hövels AM, van Haelst MM, Visser G, van Haaften G (2016) Effectiveness of whole-exome sequencing and costs of the traditional diagnostic trajectory in children with intellectual disability. Genet Med 18(9):949–956. https://doi.org/10.1038/gim.2015.200
Morgan S, Hurley J, Miller F, Giacomini M (2003) Predictive genetic tests and health system costs. CMAJ 168(8):989–991
National Centre for Human Genomics Research (2018) Technologies de la génomique pour la médecine personnalisée http://www.cea.fr/presse/Pages/dossiers/2018/genomique-medecine-personnalisee.aspx. Accessed 19 Mar 2019
Ojha RP, Thertulien R (2005) Health care policy issues as a result of the genetic revolution: implications for public health. Am J Public Health 95(3):385–388. https://doi.org/10.2105/AJPH.2003.026708
Ontario Health (Quality) (2020) Genome-wide sequencing for unexplained developmental disabilities or multiple congenital anomalies: a health technology assessment. Ont Health Technol Assess Ser 20(11):1–178 Published online 2020 Mar 6
Peyron C, Pélissier A, Béjean S (2018) Preference heterogeneity with respect to whole genome sequencing. A discrete choice experiment among parents of children with rare genetic diseases. Soc Sci Med 214:125–132. https://doi.org/10.1016/j.socscimed.2018.08.015
Pluye P, Hong QN (2014) Combining the power of stories and the power of numbers: mixed methods research and mixed studies reviews. Annu Rev Public Health 35:29–45. https://doi.org/10.1146/annurev-publhealth-032013-182440
Schwarze K, Buchanan J, Taylor JC, Wordsworth S (2018) Are whole-exome and whole-genome sequencing approaches cost-effective? A systematic review of the literature. Genet Med 20(10):1122–1130. https://doi.org/10.1038/gim.2017.24
Stark Z, Schofield D, Alam K, Wilson W, Mupfeki N, Macciocca I, Shrestha R, White SM, Gaff C (2017) Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet Med 19(8):867–874. https://doi.org/10.1038/gim.2016.221
Tsiplova K, Zur RM, Marshall CR, Stavropoulos DJ, Pereira SL, Merico D, Young EJ, Sung WWL, Scherer SW, Ungar WJ (2017) A microcosting and cost-consequence analysis of clinical genomic testing strategies in autism spectrum disorder. Genet Med 19(11):1268–1275. https://doi.org/10.1038/gim.2017.47
Turrini M, Prainsack B (2016) Beyond clinical utility: the multiple values of DTC genetics. Appl Transl Genom 8:4–8. https://doi.org/10.1016/j.atg.2016.01.008
Vass C, Rigby D, Payne K (2017) The role of qualitative research methods in discrete choice experiments. Med Decis Mak 37(3):298–313. https://doi.org/10.1177/0272989X16683934
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the topical collection on Resource Allocation in Genomic Medicine (Slade).
Rights and permissions
About this article
Cite this article
Lejeune, C., Amado, I.F. & on behalf of the DEFIDIAG study group, FHU Translad and Aviesan. Valuing genetic and genomic testing in France: current challenges and latest evidence. J Community Genet 13, 477–485 (2022). https://doi.org/10.1007/s12687-020-00503-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-020-00503-2