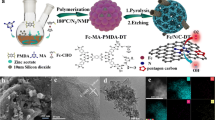
Abstract
Active and stable bifunctional catalysts for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) are required for the air electrode of rechargeable metal-air batteries. A promising catalyst could be a carbonaceous material containing transition metal ions coordinated by nitrogen and embedded in the carbon surface (metal-N–C site) as the catalytically active site, although sufficient activity and stability in such materials have not yet been attained. In this study, a systematic investigation of the effects of nanostructures on bifunctional catalytic activity was carried out to gain insight into the activity enhancement. A series of graphitic carbon materials with various concave and convex nanostructures were used as a substrate, which is advantageous for forming stable catalysts. The graphitic carbon surface was covered with a carbonaceous thin film containing the Fe–N-C site, which was prepared by iron phthalocyanine sublimation, deposition on the substrate, and decomposition. Distinct dependence of the ORR and OER activities on the nanostructures was observed. The dependence was explained by the surface Fe concentration and specific surface area, and the correlation between the OER and the concave structure was also suggested. The charge–discharge and cycling performances of a zinc-air battery with an air electrode prepared from the Fe–N-C-decorated graphitic carbon material were also tested to confirm the bifunctionality. Our study provides a useful guideline for developing active and stable bifunctional catalysts from the viewpoint of the catalyst nanostructure.
Graphical abstract








Similar content being viewed by others
References
J. Fu, R. Liang, G. Liu, A. Yu, Z. Bai, L. Yang, Z. Chen, Recent progress in electrically rechargeable zinc–air batteries. Adv. Mater. 31, 1805230 (2019). https://doi.org/10.1002/adma.201805230
X. Li, Y. Tang, H. Lv, W. Wang, F. Mo, G. Liang, C. Zhi, H. Li, Recent advances in flexible aqueous zinc-based rechargeable batteries. Nanoscale 11, 17992 (2019). https://doi.org/10.1039/C9NR06721C
X. Chen, Z. Zhou, H.E. Karahan, Q. Shao, L. Wei, Y. Chen, Recent advances in materials and design of electrochemically rechargeable zinc–air batteries. Small 14, 1801929 (2018). https://doi.org/10.1002/smll.201801929
X. Cai, L. Lai, J. Lin, Z. Shen, Recent advances in air electrodes for Zn–air batteries: electrocatalysis and structural design. Mater. Horiz. 4, 945 (2017). https://doi.org/10.1039/C7MH00358G
K. Kinoshita, Electrochemical oxygen technology (John Wiley & Sons, New York, 1992)
D.U. Lee, P. Xu, Z.P. Cano, A.G. Kashkooli, M.G. Park, Z. Chen, Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal–air batteries. J. Mater. Chem. A 4, 7107 (2016). https://doi.org/10.1039/C6TA00173D
C. Han, W. Li, H. Liu, S. Dou, J. Wang, Design strategies for developing non-precious metal based bi-functional catalysts for alkaline electrolyte based zinc–air batteries. Mater. Horiz. 6, 1812 (2019). https://doi.org/10.1039/C9MH00502A
A.J. Appleby, G. Crepy, G. Feuillade, Materials for carbon-based primary and secondary air electrodes, in Power Sources, vol. 6, ed. by D.H. Collins (Academic Press, London, 1977), pp. 549–568
G. Duperray, G. Marcellin, B. Pichon, Recent advances in secondary zinc-air batteries, in Power Sources, vol. 8, ed. by B.J. Thompson (Academic Press, London, 1981), pp. 489–511
G. Fu, Y. Tang, J. Lee, Recent advances in carbon-based bifunctional oxygen electrocatalysts for Zn−air batteries. ChemElectroChem 5, 1424 (2018). https://doi.org/10.1002/celc.201800373
M. Shao, Q. Chang, J. Dodelet, R. Chenitz, Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594 (2016). https://doi.org/10.1021/acs.chemrev.5b00462
H. Fei, J. Dong, Y. Feng, C.S. Allen, C. Wan, B. Volosskiy, M. Li, Z. Zhao, Y. Wang, H. Sun, P. An, W. Chen, Z. Guo, C. Lee, D. Chen, I. Shakir, M. Liu, T. Hu, Y. Li, A.I. Kirkland, X. Duan, Y. Huang, General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat. Catal. 1, 63 (2018). https://doi.org/10.1038/s41929-017-0008-y
J. Wang, F. Ciucci, Boosting bifunctional oxygen electrolysis for N-doped carbon via bimetal addition. Small 13, 1604103 (2017). https://doi.org/10.1002/smll.201604103
J. Maruyama, S. Maruyama, T. Fukuhara, Y. Takao, K. Miyazaki, Nanoscopic combination of edge and flat planes in the active site for oxygen reduction and evolution. Eur. J. Inorg. Chem. 2019, 4117 (2019). https://doi.org/10.1002/ejic.201900761
J. Maruyama, S. Maruyama, T. Fukuhara, K. Hanafusa, Efficient edge plane exposure on graphitic carbon fiber for enhanced flow-battery reactions. J. Phys. Chem. C 121, 24425 (2017). https://doi.org/10.1021/acs.jpcc.7b07961
J. Maruyama, T. Amano, S. Inoue, Y. Muramatsu, N. Yoshizawa, E.M. Gullikson, A carbonaceous two-dimensional lattice with FeN4 units. Chem. Commun. 54, 8995 (2018). https://doi.org/10.1039/C8CC04424D
M. Lefèvre, J.P. Dodelet, P. Bertrand, Molecular oxygen reduction in PEM fuel cells: evidence for the simultaneous presence of two active sites in Fe-based catalysts. J. Phys. Chem. B 106, 8705 (2002). https://doi.org/10.1021/jp020267f
Y. Wang, D. Adekoya, J. Sun, T. Tang, H. Qiu, L. Xu, S. Zhang, Y. Hou, Manipulation of edge-site Fe–N2 moiety on holey Fe, N codoped graphene to promote the cycle stability and rate capacity of Li–S batteries. Adv. Funct. Mater. 29, 1807485 (2019). https://doi.org/10.1002/adfm.201807485
J. Maruyama, S. Maruyama, T. Fukuhara, H. Mizuhata, S. Takenaka, A. Yoshida, K. Miyazaki, Bifunctional oxygen electrodes with highly step-enriched surface of Fe–Nx containing carbonaceous thin film. J. Electrochem. Soc. 167, 060504 (2020). https://doi.org/10.1149/1945-7111/ab7e86
J. Maruyama, K. Sumino, M. Kawaguchi, I. Abe, Influence of activated carbon pore structure on oxygen reduction at catalyst layers supported on rotating disk electrodes. Carbon 42, 3115 (2004). https://doi.org/10.1016/j.carbon.2004.07.023
J. Maruyama, T. Hasegawa, S. Iwasaki, H. Kanda, H. Kishimoto, Heat treatment of carbonized hemoglobin with ammonia for enhancement of pore development and oxygen reduction activity. ACS Sustainable Chem. Eng. 2, 493 (2014). https://doi.org/10.1021/sc400402y
V. Armel, S. Hindocha, F. Salles, S. Bennett, D. Jones, F. Jaouen, Structural descriptors of zeolitic–imidazolate frameworks are keys to the activity of Fe–N–C catalysts. J. Am. Chem. Soc. 139, 453 (2017). https://doi.org/10.1021/jacs.6b11248
S. Kubo, A. Endo, S. Yamazaki, Coral-like hierarchical carbon nanoarchitectures loaded with Rh- and Co-porphyrins as high-efficiency electrodes: effect of pore morphology on CO oxidation and oxygen reduction performance. J. Mater. Chem. A 6, 20044 (2018). https://doi.org/10.1039/C8TA05897K
M. Iqbal, Y. Bando, Z. Sun, K.C. Wu, A.E. Rowan, J. Na, B.Y. Guan, Y. Yamauchi, In search of excellence: convex versus concave noble metal nanostructures for electrocatalytic applications. Adv. Mater. 33, 2004554 (2021). https://doi.org/10.1002/adma.202004554
J. Yang, J. Tao, T. Isomura, H. Yanagi, I. Moriguchi, N. Nakashima, A comparative study of iron phthalocyanine electrocatalysts supported on different nanocarbons for oxygen reduction reaction. Carbon 145, 565 (2019). https://doi.org/10.1016/j.carbon.2019.01.022
C. Domínguez, F.J. Pérez-Alonso, M.A. Salam, S.A. Al-Thabaiti, M.A. Peña, F.J. García-García, L. Barrioa, S. Rojas, Repercussion of the carbon matrix for the activity and stability of Fe/N/C electrocatalysts for the oxygen reduction reaction. Appl. Catal. B 183, 185 (2016). https://doi.org/10.1016/j.apcatb.2015.10.043
J. Maruyama, S. Maruyama, T. Fukuhara, K. Chashiro, H. Uyama, Ordered mesoporous structure by graphitized carbon nanowall assembly. Carbon 126, 452 (2018). https://doi.org/10.1016/j.carbon.2017.10.029
J. Maruyama, T. Hasegawa, S. Iwasaki, T. Fukuhara, Y. Orikasa, Y. Uchimoto, Catalysis of vanadium ion redox reactions on carbonaceous material with Metal–N4 sites. ChemCatChem 7, 2305 (2015). https://doi.org/10.1002/cctc.201500362
M. Kawaguchi, T. Yamanaka, Y. Hayashi, H. Oda, Preparation and capacitive properties of a carbonaceous material containing nitrogen. J. Electrochem. Soc. 157, A35 (2010). https://doi.org/10.1149/1.3251293
Y. Liu, J. Bao, Z. Li, L. Zhang, S. Zhang, L. Wang, X. Niu, P. Sun, L. Xu, Large-scale defect-rich iron/nitrogen co-doped graphene-based materials as the excellent bifunctional electrocatalyst for liquid and flexible all-solid-state zinc-air batteries. J. Colloid Interface Sci. 607, 1201 (2022). https://doi.org/10.1016/j.jcis.2021.09.070
L. Gao, X. Gao, P. Jiang, C. Zhang, H. Guo, Y. Cheng, Atomically dispersed iron with densely exposed active sites as bifunctional oxygen catalysts for zinc–air flow batteries. Small 2105892 (2021). https://doi.org/10.1002/smll.202105892
G. Li, J. Yang, Y. Chen, M. Liu, X. Guo, G. Chen, B. Chang, T. Wu, X. Wang, Design and facile synthesis of highly efficient and durable bifunctional oxygen electrocatalyst Fe−Nx/C nanocages for rechargeable zinc-air batteries. ACS Appl. Mater. Interfaces 13, 54032 (2021). https://doi.org/10.1021/acsami.1c17151
V. Jose, H. Hu, E. Edison, W. Manalastas Jr., H. Ren, P. Kidkhunthod, S. Sreejith, A. Jayakumar, J.M.V. Nsanzimana, M. Srinivasan, J. Choi, J.-M. Lee, Modulation of single atomic Co and Fe sites on hollow carbon nanospheres as oxygen electrodes for rechargeable Zn–air batteries. Small Methods 2000751 (2020). https://doi.org/10.1002/smtd.202000751
M. Ma, A. Kumard, D. Wang, Y. Wang, Y. Jia, Y. Zhang, G. Zhang, Z. Yan, X. Sun, Boosting the bifunctional oxygen electrocatalytic performance of atomically dispersed Fe site via atomic Ni neighboring. Appl. Catal. B 274, 119091 (2020). https://doi.org/10.1016/j.apcatb.2020.119091
C. Yang, C. Shu, Z. Gan, C. Lai, J. Ma, W. Tang, Y. Wu, Thiocyanate ion ligand-induced atomically dispersed Fe−N−S tridoped hollow catalyst for high-performance zinc−air rechargeable batteries. Energy Fuels 34, 11620 (2020). https://doi.org/10.1021/acs.energyfuels.0c02512
C. Chen, Y. Li, D. Cheng, H. He, K. Zhou, Graphite nanoarrays-confined Fe and Co single-atoms within graphene sponges as bifunctional oxygen electrocatalyst for ultralong lasting zinc-air battery. ACS Appl. Mater. Interfaces 12, 40415 (2020). https://doi.org/10.1021/acsami.0c12801
C. Li, E. Zhou, Z. Yu, H. Liu, M. Xiong, Tailor-made open porous 2D CoFe/SN-carbon with slightly weakened adsorption strength of ORR/OER intermediates as remarkable electrocatalysts toward zinc-air batteries. Appl. Catal. B 269, 118771 (2020). https://doi.org/10.1016/j.apcatb.2020.118771
Z. Guan, X. Zhang, J. Fang, X. Wang, W. Zhu, Z. Zhuang, Fe, Ni, S, N-doped carbon materials as highly active bi-functional catalysts for rechargeable zinc-air battery. Mater. Lett. 258, 126826 (2020). https://doi.org/10.1016/j.matlet.2019.126826
Funding
This study was partly supported by Grants-In-Aid for Scientific Research (KAKENHI; grant no. 18K04870) of the Japan Society for the Promotion of Science. XAFS studies were performed with the approval of SPring-8 (Proposal No. 2018B1775).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maruyama, J., Nakajima, D., Maruyama, S. et al. Graphitic Carbon Materials with Various Nanostructures Decorated with Fe-N-C Catalytically Active Sites for Air Electrodes. Electrocatalysis 13, 219–229 (2022). https://doi.org/10.1007/s12678-022-00716-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-022-00716-8