Abstract
The development of depolarized electrodes may be considered as an improvement on economic and environmental aspects for the chemistry industry. In this regard, using a hydrogen depolarized anode (HDA) to produce protons from hydrogen oxidation could be coupled with a membrane process to concentrate valuable products. In particular, the actual standard anode used in a recent industrial lithium hydroxide electromembrane process could be replaced by an HDA. This change could lower the electric consumption and production costs. However, this evolution requires the development of an electro cell properly adaptable to the current process. In this study, we propose a proof of concept for this technology with a dedicated installation using a sulfuric acid electrolyte. Based on materials commonly used in the field of fuel cells, which share some fundamental aspects, a general understanding of hydrogen depolarized anodes will be presented. The goal is to develop an electro cell that allows anode monitoring to understand ADH operation and to compare different conditions. In this purpose, different measurements are combined to measure aspects of the ADH such as resistance, mass transfer, and kinetic overpotentials. The proposed architecture can successfully sustain the reaction at 4 kA m−1 with several mV overpotentials. But after several 10-h periods, anode operation deteriorates until reaction failure. The impact of water through the anode is identified as an important parameter to ensure long-term stability. The results obtained in this study are a first step in hydrogen depolarized anode development; they confirm the interest in this technology and identify various improvement pathways to continue its progress.
Graphical abstract
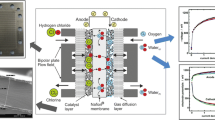
Development of a dedicated electrochemical cell allows testing the ability of a hydrogen depolarized anode to convert hydrogen to protons. The use of materials inspired by fuel cell technology was successfully adapted to a hydrogen depolarized anode. Water migration appears as a key factor, and catalytic layer flooding has to be controlled to prevent cell failure.










Similar content being viewed by others
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
I. Moussallem, J. Jörissen, U. Kunz, S. Pinnow, T. Turek, Chlor-alkali electrolysis with oxygen depolarized cathodes: history, present status and future prospects. J. Appl. Electrochem. 38, 1177–1194 (2008)
J. Clarence, A. Butler, Brine electrolysis, US Patent No. 2681884 (1950)
W. Juda, Electrochemical conversion of electrolyte solutions, US Patent No. 3124520 (1959)
W. Vielstich, Zur Energie-Ersparnis bei der Chloralkali-Elektrolyse mit Hilfe einer reversiblen Wasserstoff-Abscheidungs- oder Sauerstof-Lösungselektrode als Hauptkathode. Chem. Ing. Tech. 34, 346–349 (1962)
G. Gritzner, Operation of a cation exchange membrane electrolytic cell for producing chlorine including feeding an oxidizing gas having a regulated moisture content to the cathode, US Patent No. 4035254 (1973)
E. Yeager, P. Bindra, Sauerstoff-Verzehrkathoden für die Chloralkali-Elektrolyse. Chem. Ing. Tech. 52, 384–391 (1980)
A. Tenney, A. Pavone, Bayer-ThyssenKrupp ODC (oxygen depolarized cathode) Chlor-Alkali Technology. IHS CHEMICAL Process Economics Program Review 2015–02, (2015)
J. Jung, S. Postels, A. Bardow, Cleaner chlorine production using oxygen depolarized cathodes? A life cycle assessment. J. Clean Prod. 80, 46–56 (2014)
O.Z. Sharaf, M.F. Orhan, An overview of fuel cell technology: fundamentals and applications. Renew. Sustain. Energy Rev. 32, 810–853 (2014)
P. Symons, J.D. Genders, D. Bar, M.E. Langevin, G. Bourassa, J.F. Magnan, G. Pearse, Processes for preparing lithium hydroxide - CA2905197, Canada (2015)
G. Bourassa, G. Pearse, S.C. Mackie, M. Gladkovas, P. Symons, J. D. Genders, J.F. Magnan, Processes for preparing lithium hydroxide - US20170342573A1, USA (2017)
K. Fishel, G. Qian, G. Eisman, B.C. Benicewicz, Electrochemical hydrogen pumping in high temperature polymer electrolyte membrane fuel cells: approaches, status, and perspectives (Springer International Publishing, Cham, 2016)
H. Li, K. Lee, J. Zhang, Electrocatalytic H2 oxidation reaction in PEM fuel cell electrocatalysts and catalyst layers: fundamentals and applications (Springer, London, London, 2008)
T. Wang, H. Xie, M. Chen, A. D’Aloia, J. Cho, G. Wu, Q. Li, Precious metal-free approach to hydrogen electrocatalysis for energy conversion: from mechanism understanding to catalyst design. Nano Energy 42, 69–89 (2017)
N. Yuan, Q. Jiang, J. Li, J. Tang, A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 13, 4294–4309 (2019)
J.-H. Wee, K.-Y. Lee, S.H. Kim, Fabrication methods for low-Pt-loading electrocatalysts in proton exchange membrane fuel cell systems. J. Power Sources 165, 667–677 (2007)
N.M. Marković, P.N. Ross, Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 45, 117–229 (2002)
M.S. Wilson, J.A. Valerio, S. Gottesfeld, Low platinum loading electrodes for polymer electrolyte fuel cells fabricated using thermoplastic ionomers. Electrochim. Acta 40, 355–363 (1995)
X. Cheng, B. Yi, M. Han, J. Zhang, Y. Qiao, J. Yu, Investigation of platinum utilization and morphology in catalyst layer of polymer electrolyte fuel cells. J. Power Sources 79, 75–81 (1999)
L. Xiong, A. Manthiram, High performance membrane-electrode assemblies with ultra-low Pt loading for proton exchange membrane fuel cells. Electrochim. Acta 50, 3200–3204 (2005)
A. Ganesan, M. Narayanasamy, Ultra-low loading of platinum in proton exchange membrane-based fuel cells: a brief review. Mater. Renew. Sustain. Energy 8, 18 (2019)
R. O’Hayre, D.M. Barnett, F.B. Prinz, The triple phase boundary: a mathematical model and experimental investigations for fuel cells. J. Electrochem. Soc. 152, A439–A444 (2005)
B.C.H. Steele, Material science and engineering: the enabling technology for the commercialisation of fuel cell systems. J. Mater. Sci. 36, 1053–1068 (2001)
F. Hine, K. Murakami, Bubble effects on the solution IR drop in a vertical electrolyzer under free and forced convection. J. Electrochem. Soc. 127, 292–297 (1980)
D.E. Gray, American Institute of Physics Handbook (McGraw-Hill Book Company Inc., New York, 1957)
K. Jiao, X. Li, Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 37, 221–291 (2011)
M. Ji, Z. Wei, A review of water management in polymer electrolyte membrane fuel cells. Energies 2, 1057–1106 (2009)
T. Romero, W. Mérida, Water transport in liquid and vapour equilibrated Nafion™ membranes. J. Membr. Sci. 338, 135–144 (2009)
M. Adachi, T. Romero, T. Navessin, Z. Xie, Z. Shi, W. Mérida, S. Holdcroft, Water permeation through catalyst-coated membranes. Electrochem. Solid-State Lett. 13, B51–B54 (2010)
Q. Yan, H. Toghiani, J. Wu, Investigation of water transport through membrane in a PEM fuel cell by water balance experiments. J. Power Sources 158, 316–325 (2006)
Funding
The authors received financial support from the Fonds de recherche du Québec—Nature et technologies (FRQNT). Nicolas Sacré also received post-doctoral grant from the Mitacs and Nemaska Lithium.
Author information
Authors and Affiliations
Contributions
This manuscript was written through the contributions of all authors. All authors have approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sacré, N., Faral, M., Chenitz, R. et al. Hydrogen Depolarized Anodes with Liquid Anolyte: Proof of Concept. Electrocatalysis 13, 139–153 (2022). https://doi.org/10.1007/s12678-021-00700-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-021-00700-8