Abstract
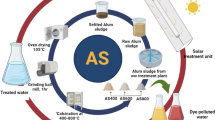
In recent years, aluminum sludge has been studied as a waste in the field of adsorption. In this study, it was prepared as a catalyst for the degradation of dye wastewater in a heterogeneous Fenton system. In this paper, a calcined aluminum sludge (CAS-700) catalyst was successfully prepared at high temperature. The physical and chemical properties of CAS-700 catalyst before and after the modification were characterized by X-ray fluorescence (XRF), X-ray diffraction (XRD), scanning electron microscope (SEM) and Brunner–Emmet–Teller (BET), and the methylene blue (MB) wastewater purification test was carried out through the heterogeneous Fenton system. The optimal experimental conditions are obtained by the response surface method and single-factor experiment. The electrode quantity of CAS-700 is 200 g/L, MB concentration is 20 mg/L, electrolyte concentration (Na2SO4) is 0.14 mol/L, voltage is 8 V, and acidic conditions are removed. The rate can reach 91.26%, which is 32% higher than the traditional two-dimensional system. In addition, CAS-700 exhibits a high catalytic efficiency ( > 88%) after six cycles. Finally, high-performance liquid chromatography-mass spectrometry (HPLC–MS) was performed to reveal the degradation pathway of MB. This not only provides a new and efficient catalyst but also provides a way for the resource utilization of aluminum sludge in water plants, in accordance with “waste for waste treatment.”
Graphical abstract











Similar content being viewed by others
References
B. Verma, C. Balomajumder, Red mud (aluminum industrial waste): An eco-friendly treatment of electroplating effluent. Can. J. Chem. Eng. 98, 2368–2380 (2020). https://doi.org/10.1002/cjce.23814
Q. Fuguo, Z. Chuanting, Review on the reclamation of aluminum-based water treatment residuals and the mechanism of pollutants removal (in Chinese with English abstract). Environmental Ence & Technology 38, 21–26 (2015)
Q. Fuguo, W. Yu, Research progress on the pollutants removal performances in water by aluminum-based water treament residuals (in Chinese with English abstract). Technology of Water Treatment. 40, 1–8 (2014). https://doi.org/10.16796/j.cnki.1000-3770.2014.06.001
Y.F. Zhou, R.J. Haynes, A comparison of water treatment sludge and red mud as adsorbents of As and Se in aqueous solution and their capacity for desorption and regeneration. Water Air Soil Pollut. 223, 5563–5573 (2012)
S. Jangkorn, S. Kuhakaew, S. Theantanoo, H. Klinla-Or, T. Sriwiriyarat, Evaluation of reusing alum sludge for the coagulation of industrial wastewater containing mixed anionic surfactants. Acta Sci. Circumst. 13, 587–594 (2011)
H.F. Wu, J.P. Wang, E.G. Duan, W.H. Hu, Y.B. Dong, G.Q. Zhang, Phosphorus removal by adsorbent based on poly-aluminum chloride sludge. Water Ence and Engineering 13, 193–201 (2020)
Y.S. Hu, Y.Q. Zhao, X.H. Zhao, J.L.G. Kumar, Comprehensive analysis of step-feeding strategy to enhance biological nitrogen removal in alum sludge-based tidal flow constructed wetlands. Biores. Technol. 111, 27–35 (2012)
H. Liu, Z. Hu, Y. Zhang, J. Zhang, S. Liang, Microbial nitrogen removal of ammonia wastewater in poly (butylenes succinate)-based constructed wetland: effect of dissolved oxygen. Appl. Microbiol. Biotechnol. 102, 9389-9398 (2018). https://doi.org/10.1007/s00253-018-9386-6
Y.Q. Zhao, A.O. Babatunde, M. Razali, F. Harty, Use of dewatered alum sludge as a substrate in reed bed treatment systems for wastewater treatment. Journal of Environmental ence and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering 43, 105–110 (2007)
Y.Q. Zhao, X.H. Zhao, A.O. Babatunde, Use of dewatered alum sludge as main substrate in treatment reed bed receiving agricultural wastewater: long-term trial. Biores. Technol. 99, 644–648 (2009)
F.-J. Chassaing, R. Mahmudov, C.D. Metcalfe, V. Yargeau, Changes to levels of microcontaminants and biological responses in rainbow trout exposed to extracts from wastewater treated by catalytic ozonation. J. Hazard. Mater. 404, 124110–124110 (2021). https://doi.org/10.1016/j.jhazmat.2020.124110
L. Lv, X. Wu, X. Han, C. Li, Amino acid modified graphene oxide for assembly of nanoparticles for wastewater treatment. Appl. Surf. Sci. (2020). https://doi.org/10.1016/j.apsusc.2020.147620
T. Gyulavari, K. Kovacs, Z. Kovacs, E. Bardos, G. Kovacs, K. Baan, K. Magyari, G. Vereb, Z. Pap, K. Hernadi, Preparation and characterization of noble metal modified titanium dioxide hollow spheres - new insights concerning the light trapping efficiency. Electrochim. Acta (2020). https://doi.org/10.1016/j.apsusc.2020.147327
N.K.A. Hamed, M.K. Ahmad, N.H.H. Hairom, A.B. Faridah, M.H. Mamat, A. Mohamed, A.B. Suriani, N. Nafarizal, F.I.M. Fazli, S.M. Fazli et al., Dependence of photocatalysis on electron trapping in Ag-doped flowerlike rutile-phase TiO2 film by facile hydrothermal method. Appl. Surf. (2020). https://doi.org/10.1016/j.apsusc.2020.147571
S. Wang, D. Li, C. Sun, S. Yang, Y. Guan, H. He, Highly efficient photocatalytic treatment of dye wastewater via visible-light-driven AgBr–Ag3PO4/MWCNTs. J. Mol. Catal. A: Chem. 383–384, 128–136 (2014)
Y. Shi, Z. Yan, Y. Xu, T. Tian, J. Zhang, J. Pang, X. Peng, Q. Zhang, M. Shao, W. Tan et al., Visible-light-driven AgBr-TiO2-palygorskite photocatalyst with excellent photocatalytic activity for tetracycline hydrochloride. J. Clean. Prod. (2020). https://doi.org/10.1016/j.jclepro.2020.124021
E.W. Moon, H.-W. Lee, J.H. Rok, J.-H. Ha, Photocatalytic inactivation of viral particles of human norovirus by Cu-doped TiO2 non-woven fabric under UVA-LED wavelengths. Science of The Total Environmen. 749, (2020). https://doi.org/10.1016/j.scitotenv.2020.141574
M. Minale, Z. Gu, A. Guadie, D.M. Kabtamu, Y. Li, X. Wang, Application of graphene-based materials for removal of tetracyclines using adsorption and photocatalytic-degradation: a review. J. Journal of Environmental Management. 276, (2020). https://doi.org/10.1016/j.jenvman.2020.111310
H. Li, L. Zhang, H. Lu, J. Ma, X. Zhou, Z. Wang, C. Yi, Macro-/nanoporous Al-doped ZnO/cellulose composites based on tunable cellulose fiber sizes for enhancing photocatalytic properties. Carbohydr. Polym. (2020). https://doi.org/10.1016/j.carbpol.2020.116873
C. Jin, Y. Lu, G. Tong, R. Che, H. Xu, Excellent microwave absorbing properties of ZnO/ZnFe2O4/Fe core-shell microrods prepared by a rapid microwave-assisted hydrothermal-chemical vapor decomposition method. Appl. Surf. Sci. (2020). https://doi.org/10.1016/j.apsusc.2020.147353
S.Y. Zuo, D.Y. Li, H.M. Xu, D.S. Xia, An integrated microwave-ultraviolet catalysis process of four peroxides for wastewater treatment: free radical generation rate and mechanism. Chem. Eng. J. 380, 12 (2020). https://doi.org/10.1016/j.cej.2019.122434
P.V. Gayathri, S. Yesodharan, E.P. Yesodharan, Microwave/persulphate assisted ZnO mediated photocatalysis (MW/PS/UV/ZnO) as an efficient advanced oxidation process for the removal of RhB dye pollutant from water. J. Environ. Chem. Eng. 7, 16 (2019). https://doi.org/10.1016/j.jece.2019.103122
A. Kadam, R. Dhabbe, D.S. Shin, K. Garadkar, J. Park, Sunlight driven high photocatalytic activity of Sn doped N-TiO2 nanoparticles synthesized by a microwave assisted method. Ceram. Int. 43, 5164–5172 (2017). https://doi.org/10.1016/j.ceramint.2017.01.039
P. Suresh, J.J. Vijaya, L.J. Kennedy, Photocatalytic degradation of textile dyeing wastewater through microwave synthesized Zr-AC, Ni-AC and Zn-AC. Trans. Nonferrous Met. Soc. Chin. 25, 4216–4225 (2015). https://doi.org/10.1016/s1003-6326(15)64072-9
N.K. Chaturvedi, S.S. Katoch, Evaluation and comparison of Fenton-like oxidation with Fenton’s oxidation for hazardous methoxyanilines in aqueous solution. J. Ind. Eng. Chem. 92, 101–108 (2020). https://doi.org/10.1016/j.jiec.2020.08.028
V. Rouge, U. von Gunten, S. Allard, Efficiency of pre-oxidation of natural organic matter for the mitigation of disinfection byproducts: electron donating capacity and UV absorbance as surrogate parameters. Water Res. (2020). https://doi.org/10.1016/j.watres.2020.116418
Y.L. Yuan, Y.Z. Wen, X.Y. Li, S.Z. Luo, Treatment of wastewater from dye manufacturing industry by coagulation. Journal of Zhejiang University - Science A: Applied Physics & Engineering 7, 340–344 (2006)
X. Xiao, C. Kangping, W. Zheng, F. Yingying, Treatment of anthraquinone dye wastewater with Fe/C electrolysis-Fenton synergistic oxidation-coagulation sedimentation-A/O process (in Chinese with English abstract). Technology of Water Treatment. 42, 55-59 (2016). https://doi.org/10.16796/j.cnki.1000-3770.2016.12.013
D.F. Viana, G.R. Salazar-Banda, M.S. Leite, Electrochemical degradation of reactive black 5 with surface response and artificial neural networks optimization models. Sep. Sci. Technol. 1–15 (2018). https://doi.org/10.1080/01496395.2018.1463264
H. Pourzamani, N. Mengelizadeh, Y. Hajizadeh, H. Mohammadi, Electrochemical degradation of diclofenac using three-dimensional electrode reactor with multi-walled carbon nanotubes. Environmental ence & Pollution Research International 25, 1–18 (2018)
X.J.R. Ni, Removal of Acid Orange 7 in simulated wastewater using a three-dimensional electrode reactor: removal mechanisms and dye degradation pathway. Chemosphere 78, 46–51 (2010)
Y.T. Wang, A.H. Lu, H.L. Zhang, W.C. Li, Synthesis of nanostructured mesoporous manganese oxides with three-dimensional frameworks and their application in supercapacitors. J. Phys. Chem. C 115, 5413–5421 (2011)
I. Kruusenberg, D. Macdonald, M. Uibu, V. Mikli, P. Walke, M. Kazemi, K. Liivand, Spent Li-ion battery graphite turned into valuable and active catalyst for electrochemical oxygen reduction. Chemsuschem (2020). https://doi.org/10.1002/cssc.202002742
Y.-J. Shih, S.-H. Huang, C.-L. Chen, C.-D. Dong, C.-P. Huang, Electrolytic characteristics of ammonia oxidation in real aquaculture water using nano-textured mono-and bimetal oxide catalysts supported on graphite electrodes. Electrochim. Acta. (2020). https://doi.org/10.1016/j.electacta.2020.136990
I.C. da Costa Soares, A.R. Lopes da Silva, E.C. Martins de Moura Santos, E.V. dos Santos, D.R. da Silva, C.A. Martinez-Huitle, Understanding the electrochemical oxidation of dyes on platinum and boron-doped diamond electrode surfaces: experimental and computational study. J. Solid State Electrochem. 24, 3245–3256 (2020). https://doi.org/10.1007/s10008-020-04813-w
J. Zambrano, H. Park, B. Min, Enhancing electrochemical degradation of phenol at optimum pH condition with a Pt/Ti anode electrode. Environ. Technol. 41, 3248–3259 (2020). https://doi.org/10.1080/09593330.2019.1649468
S. Salvestrini, A. Fenti, S. Chianese, P. Iovino, D. Musmarra, Electro-oxidation of humic acids using platinum electrodes: an experimental approach and kinetic modelling. Water (2020). https://doi.org/10.3390/w12082250
Q. Shan, C. Yidi, G. Zhenao, Y. Le, C. Dekun, Z. Zhen, Review on reaction mechanism of electro-Fenton process (in Chinese with English abstract). Environmental Ence and Management 39, 55–58 (2014)
E. Xie, L. Zheng, A. Ding, D. Zhang, Mechanisms and pathways of ethidium bromide Fenton-like degradation by reusable magnetic nanocatalysts. Chemosphere (2021). https://doi.org/10.1016/j.chemosphere.2020.127852
S. Zuo, X. Jin, X. Wang, Y. Lu, Q. Zhu, J. Wang, W. Liu, Y. Du, J. Wang, Sandwich structure stabilized atomic Fe catalyst for highly efficient Fenton-like reaction at all pH values. Applied Catalysis B-Environmental (2021). https://doi.org/10.1016/j.apcatb.2020.119551
N. Thomas, D.D. Dionysiou, S.C. Pillai, Heterogeneous Fenton catalysts: a review of recent advances. J. Hazard. Mater. 404, 124082–124082 (2021). https://doi.org/10.1016/j.jhazmat.2020.124082
K. Ghorai, A. Panda, M. Bhattacharjee, D. Mandal, A. Hossain, P. Bera, M.M. Seikh, A. Gayen, Facile synthesis of CuCr2O4/CeO2 nanocomposite: a new Fenton like catalyst with domestic LED light assisted improved photocatalytic activity for the degradation of RhB, MB and MO dyes. Appl. Surf. Sci. (2021). https://doi.org/10.1016/j.apsusc.2020.147604
Q. Wu, M.S. Siddique, W. Yu, Iron-nickel bimetallic metal-organic frameworks as bifunctional Fenton-like catalysts for enhanced adsorption and degradation of organic contaminants under visible light: kinetics and mechanistic studies. J. Hazard. Mater. 401, 123261 (2021). https://doi.org/10.1016/j.jhazmat.2020.123261
B. Song, Z. Wang, J. Li, Y. Ma, Preparation and electrocatalytic properties of kaolin/steel slag particle electrodes. Catal. Commun. (2021). https://doi.org/10.1016/j.catcom.2020.106177
M.A. Ajeel, R.I. Mahdi, M.K.T. Aroua, W. Abd Majid, Preparation and characterization of electrode from annealed nano-diamond particles with boric acid for anodic oxidation process. Electrochim. Acta (2020). https://doi.org/10.1016/j.electacta.2020.137221
J. Wang, S. Wang, Z. Zhang, C. Wang, Preparation of Cu/GO/Ti electrode by electrodeposition and its enhanced electrochemical reduction for aqueous nitrate. J. Environ. Manage. 276, 111357 (2020). https://doi.org/10.1016/j.jenvman.2020.111357
M.L.A. Ramalho, V.S. Madeira, I.L.O. Brasileiro, P.C. Fernandes, C.B.M. Barbosa, S. Arias, J.G.A. Pacheco, Synthesis of mixed oxide Ti/Fe2O3 as solar light-induced photocatalyst for heterogeneous photo-Fenton like process. Journal of Photochemistry and Photobiology a-Chemistry (2021). https://doi.org/10.1016/j.jphotochem.2020.112873
J. Liu, L. Sun, G. Li, J. Hu, Q. He, Ultrasensitive detection of dopamine via electrochemical route on spindle-like alpha-Fe2O3 mesocrystals/rGO modified GCE. Mater. Res. Bull. (2021). https://doi.org/10.1016/j.materresbull.2020.111050
Q.-Q. Xu, W. Huo, S.-S. Li, J.-H. Fang, L. Li, B.-Y. Zhang, Y.-X. Zhang, S.-W. Li, Crystal phase determined Fe active sites on Fe2O3 (gamma- and alpha-Fe2O3) yolk-shell microspheres and their phase dependent electrocatalytic oxygen evolution reaction. Appl. Surf. Sci. (2020). https://doi.org/10.1016/j.apsusc.2020.147368
Y. Gao, F.-K. Chiang, S. Li, L. Zhang, P. Wang, E.J.M. Hensen, Influence of hematite morphology on the CO oxidation performance of Au/alpha-Fe2O3. Chin. J. Catal. 42, 658–665 (2021). https://doi.org/10.1016/s1872-2067(20)63687-7
M. Ma, R. Yang, C. He, Z. Jiang, J.-W. Shi, R. Albilali, K. Fayaz, B. Liu, Pd-based catalysts promoted by hierarchical porous Al2O3 and ZnO microsphere supports/coatings for ethyl acetate highly active and stable destruction. J. Hazard. Mater. 401, 123281 (2021). https://doi.org/10.1016/j.jhazmat.2020.123281
H. Zhou, F. Zhou, S. Shi, W. Yang, Z. Song, Influence of working temperature on the electrochemical characteristics of Al2O3-coated LiNi0.8Co0.1Mn0.1O2 cathode materials for Li-ion batteries. J. Alloys Compd. (2020). https://doi.org/10.1016/j.jallcom.2020.156412
S.C. Zhang, Z.F. Liu, D. Chen, W.G. Yan, An efficient hole transfer pathway on hematite integrated by ultrathin Al2O3 interlayer and novel CuCoOx cocatalyst for efficient photoelectrochemical water oxidation. Applied Catalysis B-Environmental 277, 9 (2020). https://doi.org/10.1016/j.apcatb.2020.119197
H. Guo, Y. Wang, B, The chemical kinetics of Ca and Mg in calcite and dolomite in carbonic acid solution (in Chinese with English abstract). J. China Coal Soc. 41, 1806–1812 (2016)
J. Xiao, H.G. Wang, Determination of 11 elements in ultra basic rocks by X-ray fluorescence spectrometry (in Chinese with English abstract). Chem. Eng. 31, 24–26 (2017). https://doi.org/10.13840/j.cnki.cn21-1457/tq.2016.10.035
W.K. Han, F.Y Liu, Composition characteristics of muscovite from Xitian granite in eastern Hunan and its geological significance (in Chinese with English abstract). Low Carbon World 10, 93–95 (2020). https://doi.org/10.16844/j.cnki.cn10-1007/tk.2020.09.046
V. Vaiano, D. Barba, V. Palma, M. Colozzi, E. Palo, L. Barbato, S. Cortese, M. Miccio, Catalytic oxidative decomposition of H2S over MoS2/gamma-Al2O3. Fuel 279, 8 (2020). https://doi.org/10.1016/j.fuel.2020.118538
T.E. Bell, H. Menard, J.M.G. Carballo, R. Tooze, L. Torrente-Murciano, Hydrogen production from ammonia decomposition using Co/gamma-Al2O3 catalysts - insights into the effect of synthetic method. Int. J. Hydrogen Energy 45, 27210–27220 (2020). https://doi.org/10.1016/j.ijhydene.2020.07.090
T.K. Nevanpera, S. Pitkaaho, S. Ojala, R.L. Keiski, Oxidation of dichloromethane over Au, Pt, and Pt-Au containing catalysts supported on gamma-Al2O3 and CeO2-Al2O3. Molecules (2020). https://doi.org/10.3390/molecules25204644
A.S. Fedotov, V.I. Uvarov, M.V. Tsodikov, S. Paul, P. Simon, M. Marinova, F. Dumeignil, Dehydrogenation of cumene to alpha-methylstyrene on Re, W/gamma-Al2O3(K, Ce)/alpha-Al2O3 and Fe, Cr/gamma-Al2O3(K, Ce)/alpha-Al2O3 porous ceramic catalytic converters. Pet. Chem. 60, 1268–1283 (2020). https://doi.org/10.1134/s0965544120110080
Y. Yu, Y.Z. Ruan, Q.M. Huang, M. Zhou, Y.H. Du, R.P. Wu, Polycrystalline structure of waste slag in aluminum factory at different calcining temperature (in Chinese with English abstract). Chin. J. Struct. Chem. 22, 607–612 (2003)
L. Zen, Polycrystalline structure of waste slag in aluminum factory at different calcining temperature (in Chinese with English abstract). China Ceramics 045, 30–33 (2009)
J. Yang, C. Ma, J. Tao, J. Li, K. Du, Z. Wei, C. Chen, Z. Wang, C. Zhao, M. Ma, Optimization of polyvinylamine-modified nanocellulose for chlorpyrifos adsorption by central composite design. Carbohydr. (2020). https://doi.org/10.1016/j.carbpol.2020.116542
M.H. Kalavathy, I. Regupathi, M.G. Pillai, L.R. Miranda, Modelling, analysis and optimization of adsorption parameters for H3PO4 activated rubber wood sawdust using response surface methodology (RSM). Colloids and Surfaces B-Biointerfaces 70, 35–45 (2009). https://doi.org/10.1016/j.colsurfb.2008.12.007
M. Zbair, Z. Anfar, H.A. Ahsaine, Reusable bentonite clay: modelling and optimization of hazardous lead and p-nitrophenol adsorption using a response surface methodology approach. RSC Adv. 9, 5756–5769 (2019). https://doi.org/10.1039/c9ra00079h
K. Sen, N.K. Mondal, S. Chattoraj, J.K. Datta, Statistical optimization study of adsorption parameters for the removal of glyphosate on forest soil using the response surface methodology. Environ. Earth Sci. (2017). https://doi.org/10.1007/s12665-016-6333-7
M. Roosta, M. Ghaedi, A. Daneshfar, R. Sahraei, Experimental design based response surface methodology optimization of ultrasonic assisted adsorption of safaranin O by tin sulfide nanoparticle loaded on activated carbon. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy 122, 223–231 (2014). https://doi.org/10.1016/j.saa.2013.10.116
Y. Liu, Y. Zhao, J. Wang, Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: advances and prospects. J. Hazard. Mater. 404, 124–191 (2021). https://doi.org/10.1016/j.jhazmat.2020.124191
P. Dong, H. Wang, W. Liu, S. Wang, Y. Wang, J. Zhang, F. Lin, Y. Wang, C. Zhao, X. Duan et al., Quasi-MOF derivative-based electrode for efficient electro-Fenton oxidation. J. Hazard. Mater. 401, 123423 (2021). https://doi.org/10.1016/j.jhazmat.2020.123423
L. Zhou, Z. Hu, C. Zhang, Z. Bi, T. Jin, M. Zhou, Electrogeneration of hydrogen peroxide for electro-Fenton system by oxygen reduction using chemically modified graphite felt cathode. Sep. Purif. Technol. 111, 131–136 (2013). https://doi.org/10.1016/j.seppur.2013.03.038
X.-Y. Li, J. Xu, J.-P. Cheng, L. Feng, Y.-F. Shi, J. Ji, TiO2-SiO2/GAC particles for enhanced electrocatalytic removal of acid orange 7 (AO7) dyeing wastewater in a three-dimensional electrochemical reactor. Sep. Purif. Technol. 187, 303–310 (2017). https://doi.org/10.1016/j.seppur.2017.06.058
T. Nam, D. Patrick, D.T. Linh, L.T. Son, N.H. Chau, Electrochemical degradation and mineralization of glyphosate herbicide. Environ. Technol. 38, 2939–2948 (2017)
A. Ansari, D. Nematollahi, A comprehensive study on the electrocatalytic degradation, electrochemical behavior and degradation mechanism of malachite green using electrodeposited nanostructured beta-PbO2 electrodes. Water Res. 144, 462–473 (2018). https://doi.org/10.1016/j.watres.2018.07.056
Y. Sun, S. Zhu, W. Sun, H. Zheng, Degradation of high-chemical oxygen demand concentration pesticide wastewater by 3D electrocatalytic oxidation. J. Environ. Chem. Eng. (2019). https://doi.org/10.1016/j.jece.2019.103276
C. Huang, F. Peng, H.-J. Guo, C. Wang, M.-T. Luo, C. Zhao, L. Xiong, X.-F. Chen, X.-D. Chen, Efficient COD degradation of turpentine processing wastewater by combination of Fe-C micro-electrolysis and Fenton treatment: long-term study and scale up. Chem. Eng. J. 351, 697–707 (2018). https://doi.org/10.1016/j.cej.2018.06.139
J. Li, D. Song, K. Du, Z. Wang, C. Zhao, Performance of graphite felt as a cathode and anode in the electro-Fenton process. RSC Adv. 9, 38345–38354 (2019)
Y. Li, Y. Gao, J. Liu, The Influence Factors of phenol wastewater by complex three-dimensional electrode polarity treatment. Advanced Construction Technologies. 919-921, 2157–2160 (2014). https://doi.org/10.4028/www.scientific.net/AMR.919-921.2157
B.G. Zhang, Y.P. Hou, Z.B. Yu, Y.X. Liu, J. Huang, L. Qian, J.H. Xiong, Three-dimensional electro-Fenton degradation of rhodamine B with efficient Fe-Cu/kaolin particle electrodes: electrodes optimization, kinetics, influencing factors and mechanism. Sep. Purif. Technol. 210, 60–68 (2019). https://doi.org/10.1016/j.seppur.2018.07.084
B.B. Shao, Y.Y. Guan, Z.Y. Tian, X.H. Guan, D.L. Wu, Advantages of aeration in arsenic removal and arsenite oxidation by structural Fe(II) hydroxides in aqueous solution. Colloid Surf. A-Physicochem. Eng. Asp. 506, 703–710 (2016). https://doi.org/10.1016/j.colsurfa.2016.07.049
H. Yu, G. Liu, R. Jin, J. Zhou, Goethite-humic acid coprecipitate mediated Fenton-like degradation of sulfanilamide: the role of coprecipitated humic acid in accelerating Fe(III)/Fe(II) cycle and degradation efficiency. J. Hazard Mater. (2021). https://doi.org/10.1016/j.jhazmat.2020.124026
C. Zhang, Y. Jiang, Y. Li, Z. Hu, L. Zhou, M. Zhou, Three-dimensional electrochemical process for wastewater treatment: a general review. Chem. Eng. J. 228, 455–467 (2013). https://doi.org/10.1016/j.cej.2013.05.033
T. Zheng, Q. Wang, Z. Shi, Y. Fang, S. Shi, J. Wang, C. Wu, Advanced treatment of wet-spun acrylic fiber manufacturing wastewater using three-dimensional electrochemical oxidation. J. Environ. Sci. 50, 21–31 (2016). https://doi.org/10.1016/j.jes.2016.03.020
Y. Zhang, Z.R. Tang, X. Fu, Y.J. Xu, Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation: what advantage does graphene have over its forebear carbon nanotube. ACS Nano 5, 7426–7435 (2011)
J. Jiang, J. Zou, L. Zhu, L. Huang, Y. Zhang, Degradation of methylene blue with H2O2 activated by peroxidase-like Fe3O4 magnetic nanoparticles. J. Nanosci. Nanotechnol. 11, 4793 (2011)
C. Xue, Y. Peng, A. Chen, L. Peng, S. Luo, Drastically inhibited nZVI-Fenton oxidation of organic pollutants by cysteine: multiple roles in the nZVI/O2/hv system. J. Colloid Interface Sci. 582, 22–29 (2021). https://doi.org/10.1016/j.jcis.2020.08.036
N. Zhang, J. Bu, Y. Meng, J. Wan, L. Yuan, X. Peng, Degradation of p-aminophenol wastewater using Ti-Si-Sn-Sb/GAC particle electrodes in a three-dimensional electrochemical oxidation reactor. Appl. Organomet. Chem. 34 (2020). https://doi.org/10.1002/aoc.5612
Y. Zhang, Z. Chen, P. Wu, Y. Duan, L. Zhou, Y. Lai, F. Wang, S. Li, Three-dimensional heterogeneous Electro-Fenton system with a novel catalytic particle electrode for Bisphenol A removal. J. Hazard. Mater. (2020). https://doi.org/10.1016/j.jhazmat.2019.03.067
M.A. Rauf, M.A. Meetani, A. Khaleel, A. Ahmed, Photocatalytic degradation of methylene blue using a mixed catalyst and product analysis by LC/MS. Chem. Eng. J. 157, 373–378 (2010). https://doi.org/10.1016/j.cej.2009.11.017
M.T. Islam, M.M. Hasan, M.F. Shabik, F. Islam, Y. Nagao, M.A. Hasnat, Electroless deposition of gold nanoparticles on a glassy carbon surface to attain methylene blue degradation via oxygen reduction reactions. Electrochim. Acta (2020). https://doi.org/10.1016/j.electacta.2020.136966
Acknowledgements
The help and valuable suggestions of the co-authors are gratefully acknowledged.
Funding
Financial support from the National Natural Science Foundation of China (U1803244).
Author information
Authors and Affiliations
Contributions
Y.L Yang and C.X Ma: conceptualization, methodology, software of response surface. Y.L Yang: data curation, writing—original draft preparation. M.L Li and J.K, Wang: visualization, investigation. X.L He: supervision. J.F Li: involve in conceptualization, methodology, and software of response surface. Y.L Yang and C.X Ma: design, validate, and analysis of response surface experiments.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Calcined aluminum sludge (CAS-700) was prepared by calcining at 700 ℃ as an efficient catalyst and exhibited an excellent performance of MB degradation in a heterogeneous Fenton-like system.
2. CAS-700 catalyst exists γ-Al2O3 and Fe2O3 separately which enhanced the catalytic performance.
3. The CAS-700 catalyst from low raw material cost with a convenient preparation process.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Ma, C., He, X. et al. Calcined Aluminum Sludge as a Heterogeneous Fenton-Like Catalyst for Methylene Blue Degradation by Three-Dimensional Electrochemical System. Electrocatalysis 12, 698–714 (2021). https://doi.org/10.1007/s12678-021-00684-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-021-00684-5