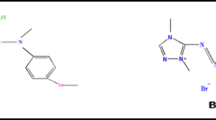
Abstract
Electrochemical treatments are attracting research attention and are being increasingly used due to their efficient degradation of organic contaminants in wastewater. In this work a home-made, expanded dimensionally stable anode (DSA) electrode (Ti–IrO2) was used owing to its excellent catalytic activity, corrosion-resistant, and chemical stability. A mixture of supporting electrolytes was used to ease the treatment process by increasing the ionic conductivity of the reaction medium, which led to reduced energy consumption, improvement of the degradation efficiency, as well as a real sample was nearly simulated. The study focused on the electrochemical degradation and degree of mineralization of the total organic carbon of the textile dye Basic Yellow 28 (BY28). The pH, supporting electrolyte mixture concentration, and current density parameters were optimized. Furthermore, to assess the efficiency of the dye degradation, the intermediates generated via electrolysis were monitored using Fourier–transform infrared, ultraviolet–visible spectroscopic, and gas chromatography–mass spectrometry techniques. The results revealed that, color removal% of the dye, chemical oxygen demand (COD)%, total organic carbon (TOC)% (degree of mineralization), current efficiency, and energy consumption values were 94.3%, 72.1%, 73.5%, 65.7%, and 41.8 kWh (g COD)−1, respectively. Cyclic voltammetry results suggest that the redox process of the BY28 dye on the surface of the Ti–IrO2 electrode can be mainly attributed to diffusion mass transport followed by- product adsorption.
Graphical abstract









Similar content being viewed by others
References
A.G. Chakinala, P.R. Gogate, A.E. Burgess, D.H. Bremner, Industrial wastewater treatment using hydrodynamic cavitation and heterogeneous advanced Fenton processing. Chem. Eng. J. 152(2–3), 498–502 (2009). https://doi.org/10.1016/j.cej.2009.05.018
H. Wang, Y. Huang, Prussian-blue-modified iron oxide magnetic nanoparticles as effective peroxidase-like catalysts to degrade methylene blue with H2O2. J. Hazard. Mater. 191(1–3), 163–169 (2011). https://doi.org/10.1016/j.jhazmat.2011.04.057
F. Ji, C. Li, J. Zhang, L. Deng, Heterogeneous photo-Fenton decolorization of methylene blue over LiFe(WO4)2 catalyst. J. Hazard. Mater. 186(2–3), 1979–1984 (2011). https://doi.org/10.1016/j.jhazmat.2010.12.089
X. bing Zhang, W. yi Dong, and W. Yang, “Decolorization efficiency and kinetics of typical reactive azo dye RR2 in the homogeneous Fe(II) catalyzed ozonation process,” Chem. Eng. J. 233, 14–23 (2013). https://doi.org/10.1016/j.cej.2013.07.098.
A.B.S. Nossol, S.M.C. Rosa, E. Nossol, A.J.G. Zarbin, P. Peralta-Zamora, Degradação fotocatalítica de corante utilizando-se nanocompósito TiO2/Óxido de grafeno. Quim. Nova 39(6), 686–690 (2016). https://doi.org/10.5935/0100-4042.20160081
L. Labiadh, M.A. Oturan, M. Panizza, N. Ben Hamadi, and S. Ammar, “Complete removal of AHPS synthetic dye from water using new electro-fenton oxidation catalyzed by natural pyrite as heterogeneous catalyst,” J. Hazard. Mater. 297, 34–41 (2015). https://doi.org/10.1016/j.jhazmat.2015.04.062.
V.M. Vasconcelos, C. Ponce-De-León, J.L. Nava, M.R.V. Lanza, Electrochemical degradation of RB-5 dye by anodic oxidation, electro-Fenton and by combining anodic oxidation-electro-Fenton in a filter-press flow cell. J. Electroanal. Chem. 765, 179–187 (2016). https://doi.org/10.1016/j.jelechem.2015.07.040
M. Punzi, A. Anbalagan, R. Aragão Börner, B.M. Svensson, M. Jonstrup, and B. Mattiasson, “Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure,” Chem. Eng. J. 270, 290–299 (2015). https://doi.org/10.1016/j.cej.2015.02.042.
A. Hethnawi, N.N. Nassar, A.D. Manasrah, G. Vitale, Polyethylenimine-functionalized pyroxene nanoparticles embedded on Diatomite for adsorptive removal of dye from textile wastewater in a fixed-bed column. Chem. Eng. J. 320, 389–404 (2017). https://doi.org/10.1016/j.cej.2017.03.057
L. M. C. Bulla, J. C. Polonio, A. L. de B. Portela-Castro, V. Kava, J. L. Azevedo, and J. A. Pamphile, “Activity of the endophytic fungi Phlebia sp. and Paecilomyces formosus in decolourisation and the reduction of reactive dyes’ cytotoxicity in fish erythrocytes,” Environ. Monit. Assess. 189(2) (2017). https://doi.org/10.1007/s10661-017-5790-0.
E. Brillas and C.A. Martínez-Huitle, “Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review,” Appl Catal B: Environ. 166–167. Elsevier, 603–643 (2015). https://doi.org/10.1016/j.apcatb.2014.11.016.
H. Olvera-Vargas, N. Oturan, E. Brillas, D. Buisson, G. Esposito, M.A. Oturan, Electrochemical advanced oxidation for cold incineration of the pharmaceutical ranitidine: Mineralization pathway and toxicity evolution. Chemosphere 117(1), 644–651 (2014). https://doi.org/10.1016/j.chemosphere.2014.09.084
H.T. Madsen, E.G. Søgaard, J. Muff, Study of degradation intermediates formed during electrochemical oxidation of pesticide residue 2,6-dichlorobenzamide (BAM) at boron doped diamond (BDD) and platinum-iridium anodes. Chemosphere 109, 84–91 (2014). https://doi.org/10.1016/j.chemosphere.2014.03.020
N. Tran, P. Drogui, T.L. Doan, T.S. Le, H.C. Nguyen, Electrochemical degradation and mineralization of glyphosate herbicide. Environ. Technol. (United Kingdom) 38(23), 2939–2948 (2017). https://doi.org/10.1080/09593330.2017.1284268
A. Dargahi, A. Ansari, D. Nematollahi, G. Asgari, R. Shokoohi, M.R. Samarghandi, Parameter optimization and degradation mechanism for electrocatalytic degradation of 2,4-diclorophenoxyacetic acid (2,4-D) herbicide by lead dioxide electrodes. RSC Adv. 9(9), 5064–5075 (2019). https://doi.org/10.1039/c8ra10105a
X.D. Tang, S. Jiao, J.J. Li, N. Hu, Deep desulfurization of kerosene by electrochemical oxidation and extraction in Mn2+/Mn3+ electrolyte. Pet. Sci. Technol. 36(7), 500–506 (2018). https://doi.org/10.1080/10916466.2018.1428624
F. Nabizadeh Chianeh and J. Basiri Parsa, “Decolorization of azo dye C.I. Acid Red 33 from aqueous solutions by anodic oxidation on MWCNTs/Ti electrodes,” Desalin. Water Treat. 57(43), 20574–20581 (2016). https://doi.org/10.1080/19443994.2015.1110716.
S. Cotillas, J. Llanos, K. Castro-Ríos, G. Taborda-Ocampo, M.A. Rodrigo, P. Cañizares, Synergistic integration of sonochemical and electrochemical disinfection with DSA anodes. Chemosphere 163, 562–568 (2016). https://doi.org/10.1016/j.chemosphere.2016.08.034
C. Massué, V. Pfeifer, X. Huang, J. Noack, A. Tarasov, S. Cap, R. Schlögl, High-performance supported iridium oxohydroxide water oxidation electrocatalysts. ChemSusChem 10(9), 1943–1957 (2017). https://doi.org/10.1002/cssc.201601817
H. Li, Q. Yu, B. Yang, Z. Li, L. Lei, Electro-catalytic oxidation of artificial human urine by using BDD and IrO2 electrodes. J. Electroanal. Chem. 738, 14–19 (2015). https://doi.org/10.1016/j.jelechem.2014.11.018
M.R. Gonçalves, I.P. Marques, J.P. Correia, Electrochemical mineralization of anaerobically digested olive mill wastewater. Water Res. 46(13), 4217–4225 (2012). https://doi.org/10.1016/j.watres.2012.05.019
E. Chatzisymeon, S. Fierro, I. Karafyllis, D. Mantzavinos, N. Kalogerakis, A. Katsaounis, Anodic oxidation of phenol on Ti/IrO2 electrode: Experimental studies. Catal. Today 151(1–2), 185–189 (2010). https://doi.org/10.1016/j.cattod.2010.02.076
J.P. Lorimer, T.J. Mason, M. Plattes, S.S. Phull, D.J. Walton, Degradation of dye effluent. Pure and Applied Chemistry 73(12), 1957–1968 (2001). https://doi.org/10.1351/pac200173121957
M. Cerón-Rivera, M.M. Dávila-Jiménez, M.P. Elizalde-González, Degradation of the textile dyes Basic yellow 28 and Reactive black 5 using diamond and metal alloys electrodes. Chemosphere 55(1), 1–10 (2004). https://doi.org/10.1016/j.chemosphere.2003.10.060
L. Yahia Cherif, I. Yahiaoui, F. Aissani-Benissad, K. Madi, N. Benmehdi, F. Fourcade, A. Amrane, “Heat attachment method for the immobilization of TiO2 on glass plates: Application to photodegradation of basic yellow dye and optimization of operating parameters, using response surface methodology,” Ind. Eng. Chem. Res. 53 (10), 3813–3819 (2014). https://doi.org/10.1021/ie403970m.
N. Daneshvar, A.R. Khataee, N. Djafarzadeh, The use of artificial neural networks (ANN) for modeling of decolorization of textile dye solution containing C. I. Basic Yellow 28 by electrocoagulation process. J. Hazard. Mater. 137(3), 1788–1795 (2006). https://doi.org/10.1016/j.jhazmat.2006.05.042
A.E. Ghaly, R. Ananthashankar, M. Alhattab, V.V. Ramakrishnan, A. Ghaly, Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Process Technol 5(1), 182 (2014). https://doi.org/10.4172/2157-7048.1000182
H. Britton, Hydrogen ions 4th edn. (1952)
A.M. Awad, N.A.A. Ghany, Electrochemical advanced oxidation of cosmetics waste water using IrO2/Ti-modified electrode. Desalin. Water Treat. 53(3), 681–688 (2015). https://doi.org/10.1080/19443994.2013.848671
N.A. Abdel Ghany, N.M. Deiab, and A.A. El-Moneim, “Anodically deposited Mn-Mo-O electrodes by direct and pulse current as novel anode materials for hydrogen production during seawater electrolysis,” Egypt. J. Chem. 52 (Special issue), 13–27 (2009). https://doi.org/10.1016/S0013-4686(02)00539-X.
N.A.A. Ghany, S. Meguro, N. Kumagai, K. Asami, K. Hashimoto, Anodically deposited Mn-Mo-Fe oxide anodes for oxygen evolution in hot seawater electrolysis. Mater. Trans. 44(10), 2114–2123 (2003). https://doi.org/10.2320/matertrans.44.2114
F. Orts, A.I. del Río, J. Molina, J. Bonastre, F. Cases, Electrochemical treatment of real textile wastewater: Trichromy Procion HEXL®. J. Electroanal. Chem. 808, 387–394 (2018). https://doi.org/10.1016/j.jelechem.2017.06.051
N. Nordin, S.F.M. Amir, Riyanto, and M.R. Othman, “Textile industries wastewater treatment by electrochemical oxidation technique using metal plate,” Int. J. Electrochem. Sci. 8(9), 11403–11415 (2013)
B.K. Körbahti, K.M. Turan, Electrochemical decolorization of reactive violet 5 textile dye using Pt/Ir electrodes. J. Turkish Chem. Soc. Sect. A Chem. 3(3), 229 (2016). https://doi.org/10.18596/jotcsa.28768
P. Kariyajjanavar, J. Narayana, Y. Arthoba Nayaka, N. Jogttappa, and Y. Arthoba Nayaka, “Studies on degradation of reactive textile dyes solution by electrochemical method,” J. Hazard. Mater. 190, 952–961 (2011). https://doi.org/10.1016/j.jhazmat.2011.04.032.
S. Chellammal, P. Kalaiselvi, P. Ganapathy, G. Subramanian, Anodic incineration of phthalic anhydride using RuO2–IrO2–SnO2–TiO2 coated on Ti anode. Arab. J. Chem. 9, S1690–S1699 (2016). https://doi.org/10.1016/j.arabjc.2012.04.030
D.Ž. Mijin, M.L. Avramov Ivić, A.E. Onjia, and B.N. Grgur, “Decolorization of textile dye CI Basic Yellow 28 with electrochemically generated active chlorine,” Chem. Eng. J. 204–205, 151–157 (2012). https://doi.org/10.1016/j.cej.2012.07.091.
L. Szpyrkowicz, C. Juzzolino, S.N. Kaul, S. Daniele, M.D. de Faveri, Electrochemical oxidation of dyeing baths bearing disperse dyes. Ind. Eng. Chem. Res. 39(9), 3241–3248 (2000). https://doi.org/10.1021/ie9908480
U. Schümann, P. Gründler, Electrochemical degradation of organic substances at PbO2 anodes: Monitoring by continuous CO2 measurements. Water Res. 32(9), 2835–2842 (1998). https://doi.org/10.1016/S0043-1354(98)00046-3
Q. Li, Q. Zhang, H. Cui, L. Ding, Z. Wei, J. Zhai, Fabrication of cerium-doped lead dioxide anode with improved electrocatalytic activity and its application for removal of Rhodamine B. Chem. Eng. J. 228, 806–814 (2013). https://doi.org/10.1016/j.cej.2013.05.064
R.M. Belal, M.A. Zayed, R.M. El-Sherif, and N.A. Abdel Ghany, “Advanced electrochemical degradation of basic yellow 28 textile dye using IrO2/Ti meshed electrode in different supporting electrolytes,” J. Electroanal. Chem. 114979 (2021). https://doi.org/10.1016/j.jelechem.2021.114979.
M.J.R. Santos, M.C. Medeiros, T.M.B.F. Oliveira, C.C.O. Morais, S.E. Mazzetto, C.A. Martínez-Huitle, S.S.L. Castro, Electrooxidation of cardanol on mixed metal oxide (RuO2-TiO2 and IrO2-RuO2-TiO2) coated titanium anodes: insights into recalcitrant phenolic compounds. Electrochim. Acta 212, 95–101 (2016). https://doi.org/10.1016/j.electacta.2016.06.145
R. Bhatnagar, H. Joshi, I.D. Mall, and V.C. Srivastava, “Electrochemical oxidation of textile industry wastewater by graphite electrodes,” J. Environ. Sci. Heal. - Part A Toxic/Hazardous Subst. Environ. Eng. 49(8), 955–966 (2014). https://doi.org/10.1080/10934529.2014.894320.
P. Kariyajjanavar, J. Narayana, Y.A. Nayaka, Degradation of textile wastewater by electrochemical method. Hydrol Curr. Res 2, 110 (2011). https://doi.org/10.4172/2157-7587.1000110
N. Jiang, Y. Wang, Q. Zhao, Z. Ye, Application of Ti/IrO2 electrode in the electrochemical oxidation of the TNT red water. Environ. Pollut. 259, 113801 (2020). https://doi.org/10.1016/j.envpol.2019.113801
E. Isarain-Chávez, M.D. Baró, E. Rossinyol, U. Morales-Ortiz, J. Sort, E. Brillas, E. Pellicer, Comparative electrochemical oxidation of methyl orange azo dye using Ti/Ir-Pb, Ti/Ir-Sn, Ti/Ru-Pb, Ti/Pt-Pd and Ti/RuO2 anodes. Electrochim. Acta 244, 199–208 (2017). https://doi.org/10.1016/j.electacta.2017.05.101
M. Panizza, G. Cerisola, Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 109(12), 6541–6569 (2009). https://doi.org/10.1021/cr9001319
F.C. Moreira, R.A.R. Boaventura, E. Brillas, and V.J.P. Vilar, “Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters,” Appl Catal. B: Environ. 202, Elsevier B.V. 217–261 (2017). https://doi.org/10.1016/j.apcatb.2016.08.037.
D.Ž Mijin, D.M. Dabić, J.M. Mirković, B.Đ Božić, B.N. Grgur, Influence of microwave irradiation on hypochlorite decolorisation of synthetic dyes. Zast. Mater. 57(1), 63–70 (2016). https://doi.org/10.5937/ZasMat1601063M
S. Singh, V.C. Srivastava, I.D. Mall, Mechanism of dye degradation during electrochemical treatment. J. Phys. Chem. C 117(29), 15229–15240 (2013). https://doi.org/10.1021/jp405289f
P.A. Carneiro, C.S. Fugivara, R.F.P. Nogueira, N. Boralle, M.V.B. Zanoni, A comparative study on chemical and electrochemical degradation of reactive blue 4 dye. Portugaliae Electrochimica Acta 21(1), 49–67 (2003). https://doi.org/10.4152/pea.200301049
G. Socrates, Infrared Characteristic Group Frequencies, 3rd edn. (John Wiley & Sons, New York, 1997)
M. Snehalatha, C. Ravikumar, N. Sekar, V.S. Jayakumar, I.H. Joe, FT-Raman, IR and UV-visible spectral investigations and ab initio computations of a nonlinear food dye amaranth. J. Raman Spectrosc. 39(7), 928–936 (2008). https://doi.org/10.1002/jrs.1938
G. Wang, Y. Liu, J. Ye, Z. Lin, X. Yang, Electrochemical oxidation of methyl orange by a Magnéli phase Ti4O7 anode. Chemosphere 241, 125084 (2020). https://doi.org/10.1016/j.chemosphere.2019.125084
S. Raghu, C.W. Lee, S. Chellammal, S. Palanichamy, C.A. Basha, Evaluation of electrochemical oxidation techniques for degradation of dye effluents-A comparative approach. J. Hazard. Mater. 171(1–3), 748–754 (2009). https://doi.org/10.1016/j.jhazmat.2009.06.063
M.G. Tavares, L.V.A. da Silva, A.M. Sales Solano, J. Tonholo, C.A. Martínez-Huitle, and C.L.P.S. Zanta, “Electrochemical oxidation of Methyl Red using Ti/Ru 0.3Ti 0.7O 2 and Ti/Pt anodes,” Chem. Eng. J. 204–205, 141–150 (2012). https://doi.org/10.1016/j.cej.2012.07.056.
Funding
This study is funded by the National Research Centre (NRC) Egypt, and Faculty of Science, Cairo University, Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Belal, R.M., Zayed, M.A., El-Sherif, R.M. et al. Electrochemical Degradation and Degree of Mineralization of the BY28 Dye in a Supporting Electrolyte Mixture Using an Expanded Dimensionally Stable Anode. Electrocatalysis 13, 26–36 (2022). https://doi.org/10.1007/s12678-021-00680-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-021-00680-9