Abstract
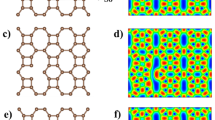
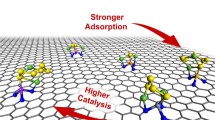
Using density functional theory calculations, we have investigated the influence of nitrogen doping in buckybowls on lithium polysulfide adsorption. Nitrogen doping in the edges of buckybowls increases the bowl depth than in the hub position. Buckybowls with pyridinic nitrogen holds lithium polysulfides with higher adsorption energies. Besides, the deeper bowl depth enhances the adsorption of lithium polysulfides. Bowl to bowl inversion is observed during the interaction with lithium polysulfides. 2 N-Pyridinic-azacorannulene exhibits better lithium polysulfide adsorption than all other 2D surfaces due to lower chemical hardness and higher bowl depth. The inference obtained in the present study can help design better scaffold material for lithium polysulfide adsorption.
Graphical abstract












Similar content being viewed by others
Code Availability
The calculations have been carried out using Gaussian 09 provided by Gaussian Inc.
References
L. Wang et al., A quantum-chemical study on the discharge reaction mechanism of lithium-sulfur batteries. J. Energy Chem. 22(1), 72–77 (2013)
Q. Liu et al., Insight on lithium polysulfide intermediates in a Li/S battery by density functional theory. RSC Adv. 7(53), 33373–33377 (2017)
M. Vijayakumar et al., Molecular structure and stability of dissolved lithium polysulfide species. Phys. Chem. Chem. Phys. 16(22), 10923–10932 (2014)
B. Wang, S.M. Alhassan, and S.T. Pantelides, Formation of large polysulfide complexes during the lithium-sulfur battery discharge. Phys. Rev. Appl. 2(3), 034004 (2014)
Y.X. Yin et al., Lithium–sulfur batteries: electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 52(50), 13186–13200 (2013)
Y.X. Yin. et al., Lithium-sulfur batteries: electrochemistry, materials, and prospects. 52, (2013)
Q. Wang et al., A shuttle effect free lithium sulfur battery based on a hybrid electrolyte. Phys. Chem. Chem. Phys. 16(39), 21225–21229 (2014)
Q. Pang et al., Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016)
X. Tao et al., Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium–sulfur battery design. Nat. Commun. 7, 11203 (2016)
M. Cheviri, S. Lakshmipathi, DFT study of chemical reactivity parameters of lithium polysulfide molecules Li2Sn(1≤n≤8) in gas and solvent phase. Computational and Theoretical Chemistry. 1202, 113323 (2021)
N. Angulakshmi, A.M. Stephan, Efficient electrolytes for lithium–sulfur batteries. Frontiers in Energy Research 3(17), (2015)
H. Pan et al., Ammonium additives to dissolve lithium sulfide through hydrogen binding for high-energy lithium–sulfur batteries. ACS Appl. Mater. Interfaces. 9(5), 4290–4295 (2017)
J.W. Park et al., Ionic liquid electrolytes for lithium–sulfur batteries. The Journal of Physical Chemistry C 117(40), 20531–20541 (2013)
M. Cheviri, S. Lakshmipathi, Cobalt phthalocyanine is a suitable scaffold for lithium polysulfide (Li2Sn n = 2–8). Chem. Phys. Lett. 739, 136942 (2020)
H.R. Jiang et al., Borophene and defective borophene as potential anchoring materials for lithium–sulfur batteries: a first-principles study. Journal of Materials Chemistry A 6(5), 2107–2114 (2018)
L.-C. Yin et al., Understanding the interactions between lithium polysulfides and N-doped graphene using density functional theory calculations. Nano Energy 25, 203–210 (2016)
L. Zhang et al., Borophene as efficient sulfur hosts for lithium–sulfur batteries: suppressing shuttle effect and improving conductivity. The Journal of Physical Chemistry C 121(29), 15549–15555 (2017)
J. Zhao et al., Phosphorene as a promising anchoring material for lithium–sulfur batteries: a computational study. Journal of Materials Chemistry A 4(16), 6124–6130 (2016)
E. Cha et al., 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li–S batteries. Nat. Nanotechnol. 13(4), 337–344 (2018)
K.C. Wasalathilake et al., Interaction between functionalized graphene and sulfur compounds in a lithium–sulfur battery – a density functional theory investigation. RSC Adv. 8(5), 2271–2279 (2018)
G.S. Yi, E.S. Sim, Y.-C. Chung, Effect of lithium-trapping on nitrogen-doped graphene as an anchoring material for lithium–sulfur batteries: a density functional theory study. Phys. Chem. Chem. Phys. 19(41), 28189–28194 (2017)
J. Chang et al., Flexible and stable high-energy lithium-sulfur full batteries with only 100% oversized lithium. Nat. Commun. 9(1), 4480 (2018)
S.P. Jand, Y. Chen, P. Kaghazchi, Comparative theoretical study of adsorption of lithium polysulfides (Li2Sx) on pristine and defective graphene. J. Power Sources 308, 166–171 (2016)
T. Maihom et al., Lithium bond impact on lithium polysulfide adsorption with functionalized carbon fiber paper interlayers for lithium–sulfur batteries. The Journal of Physical Chemistry C 122(13), 7033–7040 (2018)
S. Panigrahi, D. Umadevi, G.N. Sastry, Anomalous lithium adsorption propensity of monolayer carbonaceous materials: a density functional study. J. Chem. Sci. 128(10), 1641–1649 (2016)
F. Li, Y. Su, J. Zhao, Shuttle inhibition by chemical adsorption of lithium polysulfides in B and N co-doped graphene for Li–S batteries. Phys. Chem. Chem. Phys. 18(36), 25241–25248 (2016)
G. Li et al., Chemisorption of polysulfides through redox reactions with organic molecules for lithium–sulfur batteries. Nat. Commun. 9(1), 705 (2018)
T.Z. Hou et al., Lithium bond chemistry in lithium–sulfur batteries. Angew. Chem. Int. Ed. 56(28), 8178–8182 (2017)
I.I. Soykal et al., Highly dispersed buckybowls as model carbocatalysts for C-H bond activation. Journal of Materials Chemistry A 3(16), 8667–8675 (2015)
H. Wang et al., Advances in polar materials for lithium–sulfur batteries. Adv. Func. Mater. 28(38), 1707520 (2018)
K. Kanagaraj et al., Chiral buckybowl molecules. Symmetry 9(9), (2017)
S. Mebs et al., Experimental electron density of sumanene, a bowl-shaped fullerene fragment; comparison with the related corannulene hydrocarbon. Org. Biomol. Chem. 10(11), 2218–2222 (2012)
J. Tsuji et al., Lithium K-edge XANES spectra for lithium compounds. X-Ray Spectrom. 31(4), 319–326 (2002)
T.-C. Wu et al., Bowl-shaped fragments of C70 or higher fullerenes: synthesis, structural analysis, and inversion dynamics. Angew. Chem. Int. Ed. 52(4), 1289–1293 (2013)
A.Y. Rogachev et al., Corannulene vs. C60-fullerene in metal binding reactions: a direct DFT and X-ray structural comparison. Dalton Transactions (35), 3871–3873 (2007)
M.K. Chen et al., Highly curved bowl-shaped fragments of fullerenes: synthesis, structural analysis, and physical properties. Chem. A Eur. J. 20(2), 598–608 (2014)
V.M. Tsefrikas, L.T. Scott, Geodesic polyarenes by flash vacuum pyrolysis. Chem. Rev. 106(12), 4868–4884 (2006)
Q. Tan et al., Enantioselective synthesis of a chiral nitrogen-doped buckybowl. Nat. Commun. 3, 891 (2012)
L.G. Scanlon et al., Investigation of corannulene for molecular hydrogen storage via computational chemistry and experimentation. J. Phys. Chem. B 110(15), 7688–7694 (2006)
P.W. Rabideau, A. Sygula, Buckybowls: polynuclear aromatic hydrocarbons related to the buckminsterfullerene surface. Acc. Chem. Res. 29(5), 235–242 (1996)
A.V. Zabula et al., A main group metal sandwich: five lithium cations jammed between two corannulene tetraanion decks. Science 333(6045), 1008 (2011)
A. Reisi-Vanani, L. Alihoseini, Computational investigation of the adsorption of molecular hydrogen on the nitrogen-doped corannulene as a carbon nano-structure. Surf. Sci. 621, 146–151 (2014)
N. Ikuma et al., Internal-peripheral diosmylation of sumanene overcoming the dearomatization hurdle by the distortion of the curved π-system. Chem. Lett. 47(6), 736–739 (2018)
E. Nestoros, M.C. Stuparu, Corannulene: a molecular bowl of carbon with multifaceted properties and diverse applications. Chem. Commun. 54(50), 6503–6519 (2018)
T.J. Seiders et al., Structure/energy correlation of bowl depth and inversion barrier in corannulene derivatives: combined experimental and quantum mechanical analysis. J. Am. Chem. Soc. 123(4), 517–525 (2001)
U.D. Priyakumar, G.N. Sastry, First ab initio and density functional study on the structure, bowl-to-bowl inversion barrier, and vibrational spectra of the elusive C3v-symmetric buckybowl: sumanene, C21H12. J. Phys. Chem. A 105(18), 4488–4494 (2001)
T. Lu, F. Chen, Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. Model. 38, 314–323 (2012)
T. Lu, F. Chen, Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33(5), 580–592 (2012)
M. ang et al., Structural stability and O2 dissociation on nitrogen-doped graphene with transition metal atoms embedded: a first-principles study. AIP Advances 5(6), 067136 (2015)
M. Dieb, T., Z. Hou, K. Tsuda, Structure prediction of boron-doped graphene by machine learning. J. Chem. Phys. 148(24), 241716 (2018)
A. Akaishi et al., Structural stability and aromaticity of pristine and doped graphene nanoflakes. Jpn. J. Appl. Phys. 57, 0102BA (2018)
S.T. Skowron et al., Energetics of atomic scale structure changes in graphene. Chem. Soc. Rev. 44(10), 3143–3176 (2015)
X. Ren et al., The doping effect on the catalytic activity of graphene for oxygen evolution reaction in a lithium–air battery: a first-principles study. Phys. Chem. Chem. Phys. 17(22), 14605–14612 (2015)
M. Lalitha, S.S. Mahadevan, S. Lakshmipathi, Improved lithium adsorption in boron- and nitrogen-substituted graphene derivatives. J. Mater. Sci. 52(2), 815–831 (2017)
M. Lalitha, L. Senthilkumar, DFT study on X−·(H2O)n=1-10 (X=OH, NO2, NO3, CO3) anionic water cluster. J. Mol. Graph. Model. 54, 148–163 (2014)
V. Umadevi, L. Senthilkumar, P. Kolandaivel, Theoretical investigations on the hydrogen bonding of nitrile isomers with H2O, HF, NH3 and H2S. Mol. Simul. 39(11), 908–921 (2013)
M. Lalitha, S. Lakshmipathi, Interface energetics of [Emim]+[X]− and [Bmim]+[X]− (X=BF4, Cl, PF6, TfO, Tf2N) based ionic liquids on graphene, defective graphene, and graphyne surfaces. J. Mol. Liq. 236, 124–134 (2017)
S.F. Boys, F. Bernardi, The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19(4), 553–566 (1970)
Y. Zhang et al., Computational investigation of adsorption of molecular hydrogen on lithium-doped corannulene. J. Phys. Chem. B 110(45), 22532–22541 (2006)
S. Armaković et al., Influence of sumanene modifications with boron and nitrogen atoms to its hydrogen adsorption properties. Phys. Chem. Chem. Phys. 18(4), 2859–2870 (2016)
P. Kaewmati et al., Synthesis of triaryltriazasumanenes. Chem. Lett. 46(1), 146–148 (2016)
S. Armaković et al., Aromaticity, response, and nonlinear optical properties of sumanene modified with boron and nitrogen atoms. J. Mol. Model. 20(12), 2538 (2014)
A. Reisi-Vanani, F. Shamsali, Influence of nitrogen doping in sumanene framework toward hydrogen storage: a computational study. J. Mol. Graph. Model. 76, 475–487 (2017)
S. Armaković et al., Optical and bowl-to-bowl inversion properties of sumanene substituted on its benzylic positions; a DFT/TD-DFT study. Chem. Phys. Lett. 578, 156–161 (2013)
S. Ito, Y. Tokimaru, K. Nozaki, Benzene-fused azacorannulene bearing an internal nitrogen atom. Angew. Chem. Int. Ed. 54(25), 7256–7260 (2015)
X. Li, F. Kang, M. Inagaki, Buckybowls: corannulene and its derivatives. Small 12(24), 3206–3223 (2016)
Y. Shoji et al., Hexathioalkyl sumanenes: an electron-donating buckybowl as a building block for supramolecular materials. Chem. Sci. 8(12), 8405–8410 (2017)
S. Mukherjee et al., Adsorption and diffusion of lithium polysulfides over blue phosphorene for Li–S batteries. Nanoscale 10(45), 21335–21352 (2018)
H. Sakurai, T. Daiko, T. Hirao, A synthesis of sumanene, a fullerene fragment. Science 301(5641), 1878 (2003)
S. Fujii et al., Bowl inversion and electronic switching of buckybowls on gold. J. Am. Chem. Soc. 138(37), 12142–12149 (2016)
Acknowledgements
The authors thank the University Grants Commission, New Delhi, India, for providing funds to establish High Performance Computing facility, under the Center with Potential for Excellence in Particular Area (CPEPA) scheme [Grant 2-8/2016 (NS/PE) dated October 3, 2016].
Author information
Authors and Affiliations
Contributions
Meera Cheviri: conceptualization, methodology, formal analysis, writing—review and editing. Senthilkumar Lakshmipathi: conceptualization, methodology, formal analysis, writing—review and editing.
Corresponding author
Ethics declarations
Ethics Statement
All the ethical guidelines have been adhered to.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheviri, M., Lakshmipathi, S. Nitrogen-Doped Buckybowls as Potential Scaffold Material for Lithium-Sulfur Battery: A DFT Study. Electrocatalysis 12, 678–690 (2021). https://doi.org/10.1007/s12678-021-00678-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-021-00678-3