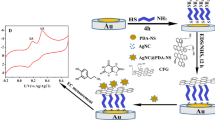
Abstract
In this study, in situ electrodeposition of gold nanoparticles (AuNPs) over polyaniline (PANI)-modified glassy carbon electrode (GCE) surface using linear sweep voltammetry (LSV) has been found to be more effective than cyclic voltammetry (CV). LSV prevents stripping of the Au atoms from the electrode surface, thus leading to an efficient loading of AuNPs on the PANI matrix. The reduction of Au over PANI-modified GCE occurs at 0.34 V and the amount of Au loaded using LSV for 15 linear sweep cycles is 54.75 × 10−9 g. Various techniques are employed to characterize the modified electrode surface. Fourier transform infrared spectroscopic studies show that the electroreduction of Au increases the quinoid moieties in PANI, and the X-ray diffraction data reveals the average crystallite size of AuNPs as 65 nm. The field emission scanning electron microscopy and atomic force microscopy analysis indicate the dispersion of spherical AuNPs over the PANI matrix. The electrochemical studies show enhanced electrocatalytic activity of the modified electrode surface at neutral pH, suitable for sensing biomolecule, dopamine (DA) amidst the interferences, ascorbic acid (AA), and uric acid (UA). The oxidation of DA reported to occur in the range of 0.270 V to 0.361 V for PANI-AuNPs based electrodes at neutral pH is lowered to 0.168 V at the modified electrode surface prepared electrochemically in the present study. The peak-to-peak separation for DA-AA and DA-UA is 108 mV and 345 mV, respectively, with the working linear range of 20–100 μM and a detection limit of 16 μM for DA. Such enhanced electrocatalytic response is attributed to a synergistic interaction between the PANI film and electrodeposited AuNPs.
Graphical Abstract



















Similar content being viewed by others
References
H. Shirakawa, E. J. Louis, A. G. MacDiarmid, C. K. Chiang, A. J. Heeger, Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 16, 578-580 (1977). https://doi.org/10.1039/C39770000578
K. Amano, H. Ishikawa, A. Kobayashi, M. Satoh, E. Hasegawa, Thermal stability of chemically synthesized polyaniline. Synth. Met. 62, 229–232 (1994). https://doi.org/10.1016/0379-6779(94)90210-0
J.G. Masters, Y. Sun, A.G. MacDiarmid, A.G. Epstein, Polyaniline: allowed oxidation states. Synth. Met. 41, 715–718 (1991). https://doi.org/10.1016/0379-6779(91)91166-8
J.C. Chiang, A.G. MacDiarmid, ‘Polyaniline’: protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 13, 193–205 (1986). https://doi.org/10.1016/0379-6779(86)90070-6
S. Bhadra, S. Chattopadhyay, N.K. Singha, D. Khastgir, Improvement of conductivity of electrochemically synthesized polyaniline. J. Appl. Polym. Sci. 108, 57–64 (2008). https://doi.org/10.1002/app.26926
M. Angelopoulos, Conducting polymers in microelectronics. IBM J. RES. & DEV. 45, 57–75 (2001). https://doi.org/10.1147/rd.451.0057
S. Palaniappan, A. John, Conjugated polymers as heterogeneous catalyst in organic synthesis. Curr. Org. Chem. 12, 98–117 (2008). https://doi.org/10.2174/138527208783330037
T. Sen, S. Mishra, N.G. Shimpi, Synthesis and sensing applications of polyaniline nanocomposites: a review. RSC Adv. 6, 42196–42222 (2016)
I. Lee, Detection of cardiac biomarkers using single polyaniline nanowire-based conductometric biosensors. Biosensors 2, 205–220 (2012). https://doi.org/10.3390/bios2020205
A.F. Diaz, J.A. Logan, Electroactive polyaniline films. J. Electroanal. Chem. 111, 111–114 (1980). https://doi.org/10.1016/S0022-0728(80)80081-7
J. Yue, A.J. Epstein, Synthesis of self-doped conducting polyaniline. J. Am. Chem. Soc. 7, 2800–2801 (1990). https://doi.org/10.1021/ja00163a051
P. N. Bartlett, P. R. Birkin, E. N. K. Wallace, Oxidation of β-nicotinamide adenine dinucleotide (NADH) at poly(aniline)-coated electrodes. J. Chem. Soc. Faraday Trans. 93, 1951-1960 (1997). https://doi.org/10.1039/A700468K
S. Tian, J. Liu, T. Zhu, W. Knoll, Polyaniline doped with modified gold nanoparticles and its electrochemical properties in neutral aqueous solution. Chem. Commun. 21, 2738–2739 (2003). https://doi.org/10.1039/B309646G
S.A. Ansari, A. Ahmed, F.K. Ferdousi, Md.A. Salam, A.A. Shaikh, H.R. Barai, N.S. Lopa, Md.M. Rahman, Conducting poly(aniline blue)-gold nanoparticles composite modified fluorine-doped tin oxide electrode for sensitive and non-enzymatic electrochemical detection of glucose. J. Electroanal. Chem. 850, 113394–113394 (2019). https://doi.org/10.1016/j.jelechem.2019.113394
K. Sadasivuni, D. Ponnamma, M. Rajan, B. Ahmed, M. Al-Maadeed, Silver Nanoparticles and Its Polymer Nanocomposites—Synthesis, Optimization, Biomedical Usage, and Its Various Applications. In: (eds) Polymer Nanocomposites in Biomedical Engineering. Lecture Notes in Bioengineering (Springer, Cham, 2019). https://doi.org/10.1007/978-3-030-04741-2_11
J.M. Kinyanjui, J. Hanks, D.W. Hatchett, A. Smith, Chemical and electrochemical synthesis of polyaniline/gold composites. J. Electrochem. Soc. 151, D113–D120 (2004). https://doi.org/10.1149/1.1808593
A. Soni, C.M. Pandey, S. Solanki, G. Sumana, One-pot synthesis of a polyaniline–gold nanocomposite and its enhanced electrochemical properties for biosensing applications. RSC Adv. 5, 45767–45774 (2015). https://doi.org/10.1039/C5RA06146F
K. Mallick, M.J. Witcomb, A. Dinsmore, M.S. Scurrell, Polymerization of aniline by auric acid: formation of gold decorated polyaniline nanoballs. Macromol. Rapid Commun. 26, 232–235 (2005). https://doi.org/10.1002/marc.200400513
D.W. Hatchett, M. Josowicz, J. Janata, Electrochemical formation of Au clusters in polyaniline. Chem. Mater. 10, 2989–2994 (1999). https://doi.org/10.1021/cm990365m
L.E.I. Ting, Preparation of novel core-shell nanoparticles by electrochemical synthesis. Trans. Nonferrous Met. Soc. CHINA. 17, 1343–1346 (2007). https://doi.org/10.1016/S1003-6326(07)60274-X
M. Hosseini, M.M. Momeni, M. Faraji, Electrochemical fabrication of polyaniline films containing gold nanoparticles deposited on titanium electrode for electro-oxidation of ascorbic acid. J. Mater. Sci. 45, 2365–2371 (2010). https://doi.org/10.1007/s10853-009-4202-4
C.C. Huang, T.C. Wen, Y. Wei, Site-selective deposition of ultra-fine Au nanoparticles on polyaniline nanofibers for H2O2 sensing. Mater. Chem. Phys. 122, 392–396 (2010). https://doi.org/10.1016/j.matchemphys.2010.03.012
S.-Y. Cui, S.-M. Park, Electrochemistry of conductive polymers XXIII: polyaniline growth studied by electrochemical quartz crystal microbalance measurements. Synth. Met. 105, 91–98 (1999)
M. Guerra-Balcázar, R. Ortega, F. Castaneda, L.G. Arriaga, J. Ledesma-Garcia, Synthesis of Au nanoparticles/polyaniline composites by electroreduction for glucose oxidation. Int. J. Electrochem. Sci. 6, 4667–4675 (2011)
V. Mazeikoa, A. Kausaite-Minkstimienea, A. Ramanavicienea, Z. Balevicius, A. Ramanavicius, Gold nanoparticle and conducting polymer-polyaniline-based nanocomposites for glucose biosensor design. Sens. Actuators B Chem. 189, 187–193 (2013). https://doi.org/10.1016/j.snb.2013.03.140
Z. Miao, P. Wang, A. Zhong, M. Yang, Q. Xu, S. Hao, X. Hu, Development of a glucose biosensor based on electrodeposited gold nanoparticles-polyvinylpyrrolidone-polyaniline nanocomposites. J. Electroanal. Chem. 756, 153–160 (2015). https://doi.org/10.1016/j.jelechem.2015.08.025
A. Wang, J. Feng, Y. Li, In-situ decorated gold nanoparticles on polyaniline with enhanced electrocatalysis toward dopamine. Microchim. Acta. 171, 431–436 (2010). https://doi.org/10.1007/s00604-010-0452-8
M.O. Shaikh, B. Srikanth, P.-Y. Zhu, C.-H. Chuang, Impedimetric immunosensor utilizing polyaniline/gold nanocomposite-modified screen-printed electrodes for early detection of chronic kidney disease. Sensors (Basel). 19, 3990–3402 (2019). https://doi.org/10.3390/s19183990
R.S. Saberi, S. Shahrokhian, G. Marrazza, Amplified electrochemical DNA sensor based on polyaniline film and gold nanoparticles. Electroanalysis 25, 1373–1380 (2013). https://doi.org/10.1002/elan.201200434
M. Sheffer, D. Mandler, Control of locally deposited gold nanoparticle on polyaniline films. Electrochim. Acta. 54, 2951–2956 (2009). https://doi.org/10.1016/j.electacta.2008.12.027
E. Spain, T.E. Keyes, R.J. Forster, Vapour phase polymerised polyaniline- gold nanoparticle composites for DNA detection. J. Electroanal. Chem. 711, 38–44 (2013). https://doi.org/10.1016/j.jelechem.2013.08.023
D.W. Hatchett, T. Quy, N. Goodwin, N.M. Millick, In situ reduction of Au, Pd, and Pt metal precursors in polyaniline: electrochemistry of variable metal content polymer/metal composites in alkaline solution. Electrochim. Acta. 251, 699–709 (2017). https://doi.org/10.1016/j.electacta.2017.08.120
R. M. Wightman, C. Amatore, R. C. Engstrom, P. D. Hale, E. W. Kristensen, W. G. Kuhr, L. J. May, Real-time Characterization of Dopamine Overflow and Uptake in the Rat Striatum. Neuroscience. 25, 513-523 (1998). https://doi.org/10.1016/0306-4522(88)90255-2
R.M. Wightman, L.J. May, A.C. Micheal, Detection of dopamine dynamics in the brain. Anal. Chem. 60, 769A-779A (1988). https://doi.org/10.1021/ac00164a001
R.R. Gonzalez, R.F. Fernandez, J.L.M. Vidal, A.G. Frenich, M.L.G. Perez, Development and validation of an ultra-high-performance liquid chromatography-tandem mass-spectrometry (UHPLC-MS/MS) method for the simultaneous determination of neurotransmitters in rat brain samples. J. Neurosci Methods. 198, 187–194 (2011). https://doi.org/10.1016/j.jneumeth.2011.03.023
H. Aldewachi, T. Chalati, M.N. Woodroofe, N. Bricklebank, B. Sharrack, P. Gardiner, Gold nanoparticle-based colorimetric biosensors. Nanoscale. 10, 18–33 (2018). https://doi.org/10.1039/C7NR06367A
H. Zhao, H. Mu, Y. Bai, H. Yu, H. Yu, A rapid method for the determination of dopamine in porcine muscle by pre-column derivatization and HPLC with fluorescence detection. J. Pharm. Anal. 1, 208–212 (2011). https://doi.org/10.1016/j.jpha.2011.04.003
G. Selvolini, C. Lazzarini, G. Marrazza, Electrochemical nanocomposite single-use sensor for dopamine detection. Sensors (Basel). 19, 3097–3098 (2019). https://doi.org/10.3390/s19143097
G. Fabregat, J. Casanovas, E. Redondo, E. Armelin, C. Alemán, A rational design for the selective detection of dopamine using conducting polymers. Phys. Chem. Chem. Phys. 16, 7850–7861 (2014). https://doi.org/10.1039/C4CP00234B
Lei Zhang, Xiue Jiang, Attachment of gold nanoparticles to glassy carbon electrode and its application for the voltammetric resolution of ascorbic acid and dopamine. J. Electroanal. Chem. 583, 292–299 (2005). https://doi.org/10.1016/j.jelechem.2005.06.014
N.F. Atta, M.F. El-Kady, A. Galal, Simultaneous determination of catecholamines, uric acid and ascorbic acid at physiological levels using poly(N-methylpyrrole)/Pd-nanoclusters sensor. Anal. Biochem. 400, 78–88 (2010). https://doi.org/10.1016/j.ab.2010.01.001
J. Mathiyarasu, S. Senthilkumar, K.L.N. Phani, V. Yegnaraman, Selective detection of dopamine using a functionalised polyaniline composite electrode. J. Appl. Electrochem. 35, 513–519 (2005). https://doi.org/10.1007/s10800-005-0914-6
X. Feng, C. Mao, G. Yang, W. Hou, J.J. Zhu, Polyaniline/Au composite hollow spheres: synthesis, characterization, and application to the detection of dopamine. Langmuir 22, 4384–4389 (2006). https://doi.org/10.1021/la053403r
M.I. Prodromidis, A.B. Florou, S.M. Tzouwara-Karayanni, M.I. Karayannis, The importance of surface coverage in the electrochemical study of chemically modified electrodes. Electroanalysis. 12, 1498–1501 (2000). https://doi.org/10.1002/1521-4109(200012)12:18%3c1498::AID-ELAN1498%3e3.0.CO;2-Y
S. Trasatti, O.A. Petrii, Real surface area measurements in Electrochemistry. Pure Appl. Chem. 63, 711–734 (1991). https://doi.org/10.1351/pac199163050711
S. Tawde, D. Mukesh, J.V. Yakhmi, Redox behavior of polyaniline as influenced by aromatic sulphonate anions: cyclic voltammetry and molecular modelling. Synth. Met. 125, 401–413 (2002). https://doi.org/10.1016/S0379-6779(01)00483-0
M. Matsushita, H. Kuramitz, S. Tanaka, Electrochemical oxidation for low concentration of aniline in neutral pH medium: application to the removal of aniline based on the electrochemical polymerization on a carbon fiber. Environ. Sci. Technol. 39, 3805–3810 (2005). https://doi.org/10.1021/es040379f
J.M. Kinyanjui, D.W. Hatchett, Chemical synthesis of a polyaniline/gold composite using tetrachloroaurate. Chem. Mater. 16, 3390–3398 (2004). https://doi.org/10.1021/cm049478i
M. Trchova, I. Sedenkova, E.N. Konyushenko, J. Stejskal, P. Holler, G. CÄiric-Marjanović, Evolution of Polyaniline Nanotubes: The Oxidation of Aniline in Water. J. Phys. Chem. B 110, 9461-9468 (2006). https://doi.org/10.1021/jp057528g
M. Etesami, F.S. Karoonian, N. Mohamed, Electrochemical deposition of gold nanoparticles on pencil graphite by fast scan cyclic voltammetry. J. Chin. Chem. Soc. 58, 688–693 (2011)
A. Patterson, The Scherrer formula for X-ray particle size determination. Phys. Rev. 56, 978–982 (1939). https://doi.org/10.1103/PhysRev.56.978
H. Matsumoto, M. Yanagida, K. Tanimoto, M. Nomura, Y. Kitagawa, Highly conductive room temperature molten salts based on small trimethylalkylammonium cations and bis (trifluoromethylsulfonyl) imide. Chem. Lett. 29(8), 922–923 (2000)
L. Yang, S. Liu, Q. Zhang, F. Li, Simultaneous electrochemical determination of dopamine and ascorbic acid using AuNPs@polyaniline core–shell nanocomposites modified electrode. Talanta 89, 136–141 (2012). https://doi.org/10.1016/j.talanta.2011.12.002
E. Laviron, The use of linear potential sweep voltammetry and of a.c. voltammetry for the study of the surface electrochemical reaction of strongly adsorbed systems and of redox modified electrodes, J. Electroanal. Chem. 100, 263-270 (1979). https://doi.org/10.1016/S0022-0728(79)80167-9
A.J. Wang, J.J. Feng, Y.F. Li, J.L. Xi, W.J. Dong, In-situ decorated gold nanoparticles on polyaniline with enhanced electrocatalysis toward dopamine. Microchim. Acta 171, 431–436 (2010). https://doi.org/10.1007/s00604-010-0452-8
W. Chu, Q. Zhou, S. Li, W. Zhao, N. Li, J. Zheng, Oxidation and sensing of ascorbic acid and dopamine on self-assembled gold nanoparticles incorporated within polyaniline film. Appl. Surf. Sci. 353, 425–432 (2015). https://doi.org/10.1016/j.apsusc.2015.06.141
S. Mahalakshmi, V. Sridevi, Conducting, crystalline and electroactive polyaniline-Au nanocomposites through combined acid and oxidative doping pathways for biosensing applications: Detection of dopamine. Mater. Chem. Phys. 235, 121728–121741 (2019). https://doi.org/10.1016/j.matchemphys.2019.121728
A. Stoyanova, S. Ivanov, V. Tsakova, A. Bund, Au nanoparticle–polyaniline nanocomposite layers obtained through layer-by-layer adsorption for the simultaneous determination of dopamine and uric acid. Electrochim. Acta 56, 3693–3699 (2011). https://doi.org/10.1016/j.electacta.2010.09.054
Y. Zhang, L. Lin, Z. Feng, J. Zhou, Z. Lin, Fabrication of a PANI/Au nanocomposite modified nanoelectrode for sensitive dopamine nanosensor design. Electrochim. Acta 55, 265–270 (2009). https://doi.org/10.1016/j.electacta.2009.08.048
M.J. Song, S.K. Lee, J.H. Kim, D.S. Lim, Dopamine sensor based on a boron-doped diamond electrode modified with a polyaniline/Au nanocomposite in the presence of ascorbic acid. Anal. Sci. 28, 583–587 (2012). https://doi.org/10.2116/analsci.28.583
S. Prakash, C.R.K. Rao, M. Vijayan, Polyaniline–polyelectrolyte–gold (0) ternary nanocomposites: synthesis and electrochemical properties. Electrochim. Acta. 54, 5919–5927 (2009). https://doi.org/10.1016/j.electacta.2009.05.059
Acknowledgements
The authors would like to acknowledge the Science Instrumentation Centre, Lady Doak College, Madurai established and funded by University Grants Commission and Department of Science and Technology, India, in carrying out the present work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahalakshmi, S., Sridevi, V. In Situ Electrodeposited Gold Nanoparticles on Polyaniline-Modified Electrode Surface for the Detection of Dopamine in Presence of Ascorbic Acid and Uric Acid. Electrocatalysis 12, 415–435 (2021). https://doi.org/10.1007/s12678-021-00665-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-021-00665-8