Abstract
An ultrasensitive electrochemical sensor to detect arsenic (III), a highly toxic heavy metal ion, has been successfully developed. The sensing platform composed of L-cysteine (Lcyst) molecules conjugated to reduced lipoic acid (rLA) capped gold nanoparticles and exhibited an indirect band gap energy (Eg) of 1.96 eV. This is a characteristic of a semiconducting material; metals and semiconducting nanomaterials possess good electrochemical properties due to their trivial band gap energies. Surface ligation of the gold nanoparticles with rLA and Lcyst did not only prevent the nanoparticle agglomeration but also improved the susceptibility of the sensor to bind efficiently to heavy metal ions of interest. The electrochemical properties of the sensing platform were demonstrated by a reversible redox electron transfer reaction of the gold oxides due to the presence of gold nanoparticle clusters on the screen-printed carbon electrode surface. The sensor parameters such as the scan rate, pH and the deposition potential were optimised. The sensor was capable of detecting the arsenic (III) ions at the formal peak potential (Epa°′) of −0.307 ± 0.02 V. Subsequently, the sensor demonstrated a low limit of detection with the magnitude of 3 ppb (i.e. 3SD/SLOPE). The dynamic linear range of the developed sensing platform was between 3 and 25 ppb. The sensor was used to detect As(III) in ground water and demonstrated excellent recoveries in spiked samples.
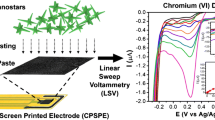
Graphical abstract
The AuNP-rLA-Lcyst sensing platform fabricated for the detection of As(III) heavy metal ion using the square-wave anodic stripping voltammetry.














Similar content being viewed by others
References
S. Dutta, G. Strack, P. Kurup, “Gold nanostar electrodes for heavy metal detection,” Sensors Actuators. B Chem. 281(September 2018), 383–391 (2019)
A. Karthika, S. Selvarajan, P. Karuppasamy, A. Suganthi, and M. Rajarajan, “A novel highly efficient and accurate electrochemical detection of poisonous inorganic Arsenic (III) ions in water and human blood serum samples based on SrTiO3/β-cyclodextrin composite,” J. Phys. Chem. Solids 127(November 2018), 11–18 (2019)
K. Gibbon-Walsh, P. Salaün, C.M.G. van den Berg, Arsenic speciation in natural waters by cathodic stripping voltammetry. Anal. Chim. Acta 662(1), 1–8 (2010)
M. Rajkumar, S. Thiagarajan, S.M. Chen, Electrochemical detection of arsenic in various water samples. Int. J. Electrochem. Sci. 6(8), 3164–3177 (2011)
L. Bu, J. Liu, Q. Xie, S. Yao, Anodic stripping voltammetric analysis of trace arsenic(III) enhanced by mild hydrogen-evolution at a bimetallic Au–Pt nanoparticle modified glassy carbon electrode. Electrochem. commun. 59, 28–31 (2015)
A. Buffa, D. Mandler, Arsenic(III) detection in water by flow-through carbon nanotube membrane decorated by gold nanoparticles. Electrochim. Acta 318, 496–503 (2019)
L. Cui, J. Wu, H. Ju, Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 63, 276–286 (2015)
T. Gu et al., “Dual-signal anodic stripping voltammetric determination of trace arsenic ( III ) at a glassy carbon electrode modi fi ed with internal-electrolysis deposited gold nanoparticles,” Electrochem. Commun. 33, 43–46 (2013)
P. Salaün, K.B. Gibbon-Walsh, G.M.S. Alves, H.M.V.M. Soares, C.M.G. van den Berg, Determination of arsenic and antimony in seawater by voltammetric and chronopotentiometric stripping using a vibrated gold microwire electrode. Anal. Chim. Acta 746, 53–62 (2012)
M.A. Kamyabi, A. Aghaei, Electromembrane extraction coupled to square wave anodic stripping voltammetry for selective preconcentration and determination of trace levels of As ( III ) in water samples. Electrochim. Acta 206, 192–198 (2016)
A. Sugunan, C. Thanachayanont, J. Dutta, J. G. Hilborn, “Heavy-metal ion sensors using chitosan-capped gold nanoparticles,” Sci. Technol. Adv. Mater. 6(3–4 SPEC. ISS.), 335–340 (2005)
L. Xiao, G.G. Wildgoose, R.G. Compton, Sensitive electrochemical detection of arsenic (III) using gold nanoparticle modified carbon nanotubes via anodic stripping voltammetry. Anal. Chim. Acta 620(1–2), 44–49 (2008)
M. Korolczuk, M. Ochab, I. Rutyna, Determination of As ( III ) by anodic stripping voltammetry following double deposition and stripping steps at two gold working electrodes. Talanta 144, 517–521 (2015)
A.D. Robles, S.N. Vettorelo, M. Gerpe, F. Garay, The electrochemical reaction mechanism of arsenic on gold analyzed by anodic stripping Square-wave voltammetry. Electrochim. Acta 227, 447–454 (2017)
T. K. Sau, A. Pal, N. R. Jana, Z. L. Wang, T. Pal, “Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles,” pp. 257–261 (2001)
J. Zha, C. Dong, X. Wang, X. Zhang, X. Xiao, X. Yang, “Green synthesis and characterization monodisperse gold nanoparticles using Ginkgo Biloba leaf extract,” Opt. - Int. J. Light Electron Opt. (2017)
T. J. Macdonald et al., “Thiol-capped gold nanoparticles swell-encapsulated into polyurethane as powerful antibacterial surfaces under dark and light conditions,” no. October, pp. 1–11 (2016)
J.M. Devi, Journal of Molecular Graphics and Modelling Simulation studies on structural and thermal properties of alkane thiol capped gold nanoparticles. J. Mol. Graph. Model. 74, 359–365 (2017)
G. Bodelón, C. Costas, J. Pérez-juste, I. Pastoriza-santos, L.M. Liz-marzán, Nano Today Gold nanoparticles for regulation of cell function and behavior. Nano Today 13, 40–60 (2017)
R. Gupta, B. Rai, Effect of size and surface charge of gold nanoparticles on their skin permeability : a molecular dynamics study, Nat. Publ. Gr., no. October 2016, pp. 1–13 (2017)
J. Santhoshkumar, S. Rajeshkumar, S.V. Kumar, Phyto-assisted synthesis, characterization and applications of gold nanoparticles—a review. Biochem. Biophys. Reports 11(June), 46–57 (2017)
B. D. Chithrani, A. A. Ghazani, W. C. W. Chan, Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells,” (2006)
F.B.Z. Jingyue, “SYNTHESIS OF GOLD NANOPARTICLES VIA CHEMICAL REDUCTION METHODS,” Brno, Czech Repub. (2015)
F.R. Tentor et al., International Journal of Biological Macromolecules Scaffolds based on chitosan/pectin thermosensitive hydrogels containing gold nanoparticles. Int. J. Biol. Macromol. 102, 1186–1194 (2017)
P. Mineo, A. Abbadessa, A. Mazzaglia, A. Gulino, V. Villari, N. Micali, Dyes and Pigments Gold nanoparticles functionalized with PEGylate uncharged porphyrins. Dye. Pigment. 141, 225–234 (2017)
M.G. Trachioti, A.E. Karantzalis, J. Hrbac, M. I. Prodromidis, Low-cost screen-printed sensors on-demand: instantly prepared sparked gold nanoparticles from eutectic Au/Si alloy for the determination of arsenic at the sub-ppb level, Sensors Actuators, B Chem. 281(July 2018), 273–280 (2019)
B.Y. Lu, J.Y. Chang, Rapid and irreversible reduction of protein disulfide bonds. Anal. Biochem. 405(1), 67–72 (2010)
J. Messens et al., How thioredoxin can reduce a buried disulphide bond. J. Mol. Biol. 339(3), 527–537 (2004)
M.A. Chowdury, N. Walji, A. Mahmud, B.D. Macdonald, Paper-based microfluidic device with a gold nanosensor to detect arsenic contamination of groundwater in Bangladesh, micromachines Artic. (2017)
D.A. El-hag, A.A. Dahab, Identification and characterisation of disulphide bonds in therapeutic proteins by using Raman Spectroscopy Identification and characterisation of disulphide bonds in therapeutic proteins by using Raman Spectroscopy. Adv J Parm Life Sci Res 4(3), 50–59 (2016)
P. Bazylewski, R. Divigalpitiya, G. Fanchini, In situ Raman spectroscopy distinguishes between reversible and irreversible thiol modifications in l-cysteine. RSC Adv. 7(5), 2964–2970 (2017)
T. Ghaly, B.E. Wildt, P.C. Searson, Electrochemical release of Fluorescently labeled thiols from patterned Gold surfaces. Langmuir 26(3), 1420–1423 (2010)
M. Homberger, U. Simon, On the application potential of gold nanoparticles in nanoelectronics and biomedicine, Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 368(1915), 1405–1453 (2010)
J. McNulty, V. Krishnamoorthy, D. Amoroso, M. Moser, Tris(3-hydroxypropyl)phosphine (THPP): a mild, air-stable reagent for the rapid, reductive cleavage of small-molecule disulfides. Bioorganic Med. Chem. Lett. 25(19), 4114–4117 (2015)
C. Zhou, W. Qi, E.N. Lewis, J.F. Carpenter, Concomitant Raman spectroscopy and dynamic light scattering for characterization of therapeutic proteins at high concentrations. Anal. Biochem. 472, 7–20 (2015)
B.A. Kolesov, “Raman spectra of crystalline secondary amides,” Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 179, 216–220 (2017)
E.U. Stolarczyk, K. Stolarczyk, M. Łaszcz, M. Kubiszewski, A. Leś, O. Michalak, Pemetrexed conjugated with gold nanoparticles—synthesis, characterization and a study of noncovalent interactions. Eur. J. Pharm. Sci. 109(June), 13–20 (2017)
A. Dey, S. Sundarapandian, Green synthesis of gold nanoparticles and evaluation of its cytotoxic property against colon cancer cell line, Res. J. Life Sci. Bioinformatics, Pharm. Chem. Sci., no. November (2018)
D. Zare, K. Khoshnevisan, M. Barkhi, H.V. Tahami, Fabrication of capped gold nanoparticles by using various amino acids. J. Exp. Nanosci. 9(9), 957–965 (2014)
S.D. Korlann, A.E. Riley, B.L. Kirsch, B.S. Mun, S.H. Tolbert, Chemical tuning of the electronic properties in a periodic surfactant-templated nanostructured semiconductor. J. Am. Chem. Soc. 127(36), 12516–12527 (2005)
V.G. Yarzhemsky, E.N. Murav’Ev, M.A. Kazaryan, Y.A. Dyakov, Electronic structure of gold nanoparticles, Inorg. Mater. 48(11), 1075–1077 (2012)
S.Y. Rhieu, V. Reipa, Tuning the size of gold nanoparticles with repetitive oxidation-reduction cycles. Am. J. Nanomater. 3(1), 15–21 (2015)
A. Garc, C.W. Foster, D.A.C. Brownson, C.E. Banks, Determination of the electrochemical area of screen-printed electrochemical sensing platforms, pp. 1–10 (2018)
P. Kumar, P. Devi, R. Jain, A. Saini, R. Noetzel, Electrochemical detection of trace arsenic (III) by functionalized In0.38Ga0.62N/Si(1 1 1) electrode. Mater. Lett. 236, 587–590 (2019)
X. Xuan, M.F. Hossain, J.Y. Park, A fully integrated and miniaturized heavy-metal-detection sensor based on micro-patterned reduced graphene oxide. Sci. Rep. 6(April), 1–8 (2016)
S. Saha, P. Sarkar, Differential pulse anodic stripping voltammetry for detection of As ( III ) by Chitosan-Fe ( OH ) 3 modi fi ed glassy carbon electrode: a new approach towards speciation of arsenic, Talanta 158, 235–245 (2016)
A. Ksakas, K. Tanji, B. El Bali, M. Taleb, A. Kherbeche, Removal of Cu ( II ) ions from aqueous solution by adsorption using natural clays : kinetic and thermodynamic studies removal of Cu (II) ions from aqueous solution by adsorption using natural clays: kinetic and thermodynamic studies, J. Mater. Environ. Sci., no. January (2018)
N. Samadi, R. Ansari, B. Khodavirdilo, Removal of copper ions from aqueous solutions using polymer derivations of poly (styrene-alt- maleic anhydride), Egypt. J. Pet., pp. 375–389 (2017)
J. Wang, H. Tao, T. Lu, Y. Wu, Adsorption enhanced the oxidase-mimicking catalytic activity of octahedral-shape Mn3O4 nanoparticles as a novel colorimetric chemosensor for ultrasensitive and selective detection of arsenic. J. Colloid Interface Sci. 584, 114–124 (2021)
P.J. Babu, M. Doble, Albumin capped carbon-gold (C-Au) nanocomposite as an optical sensor for the detection of Arsenic(III). Opt. Mater. (Amst) 84(January), 339–344 (2018)
H. Li et al., Facile synthesis of magnetic ionic liquids/gold nanoparticles/porous silicon composite SERS substrate for ultra-sensitive detection of arsenic, Appl. Surf. Sci. 545(August 2020), 148992 (2021)
B. Zheng et al., Rapid colorimetric detection of arsenic (III) by glutathione functionalized gold nanoparticles based on RGB extracting system, Opt. Laser Technol. 133(August 2020), 1–6 (2021)
T. Agustiany, M. Khalil, Y. Einaga, P. K. Jiwanti, and T. A. Ivandini, Stable iridium-modified boron-doped diamond electrode for the application in electrochemical detection of arsenic (III), Mater. Chem. Phys. 244(October 2019), 1–7 (2020)
A.R. Sadrolhosseini, S. Shafie, S.A. Rashid, M.A. Mahdi, Surface plasmon resonance measurement of arsenic in low concentration using polypyrrole-graphene quantum dots layer, Meas. J. Int. Meas. Confed., no. February (2020)
Funding
The project was sponsored by the National Research Foundation (NRF) of South Africa through the National Innovation Centre of the Advance Materials Division at Mintek.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jijana, A.N., Mphuthi, N., Shumbula, P. et al. The Ultra-sensitive Electrochemical Detection of As(III) in Ground Water Using Disposable L-cysteine/Lipoic Acid Functionalised Gold Nanoparticle Modified Screen-Printed Electrodes. Electrocatalysis 12, 310–325 (2021). https://doi.org/10.1007/s12678-021-00658-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-021-00658-7