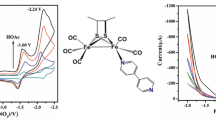
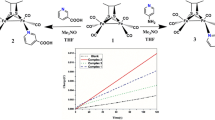
Abstract
Ligand-substitution reaction of [(μ-S2C4N2H2)Fe2(CO)6] (1) generated a normal mono-substituted diiron dithiolate derivative [(μ-S2C4N2H2)Fe2(CO)5(PMe3)] (1P) and a simpler hexacoordinate mononuclear compound [(μ-S2C4N2H2)Fe([CO)2(PMe3)2] (2) via distinct reactivity pathways under identical conditions. 1P and 2 could act as the electrochemically functional mimics of the [2Fe] sub-cluster and the distal Fe moiety of the active site of [FeFe] hydrogenase. The electrochemical investigations showed that 2 catalyzed the production of hydrogen from weak acid (acetic acid, HOAc) via two catalytic processes with an initial metal-orbital based reduction. In contrast, the electrocatalytic reaction of 2 with stronger acid (trifluoroacetic acid, TFA) occurred via an initial ligand protonation, and proceeded through different pathways that involved distinct oxidation states of the catalyst. The most striking result obtained in this study was that the hydrogen formation by 2 from TFA occurred at a relatively low overpotential as small as −0.28 V. It could be rationalized by the exclusive employment of iron(II) and iron(I) redox levels in the catalytic cycle, which was consistent with the enzymatic process. The observations might shed light on some aspects of ways by which the model compounds catalyzed the reduction of protons.

Graphical Abstract










Similar content being viewed by others
References
C. Tard, C.J. Pickett, Structural and functional analogues of the active sites of the [Fe]-, [NiFe]-, and [FeFe]-hydrogenases. Chem. Rev. 109, 2245 (2009)
W. Lubitz, H. Ogata, O. Rüdiger, E. Reijerse, Hydrogenases. Chem. Rev. 114, 4081 (2014)
J.W. Peters, W.N. Lanzilotta, B.J. Lemon, L.C. Seefeldt, X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282, 1853 (1998)
Y. Nicolet, C. Piras, P. Legrand, E.C. Hatchikian, J.C. Fontecilla-Camps, Desulfovibrio desulfuricans Iron hydrogenase: The structure shows unusual coordination to an active site Fe binuclear center. Structure 7, 13 (1999)
A.L. De Lacey, C. Stadler, C. Cavazza, E.C. Hatchikian, V.M. Fernandez, FTIR characterization of the active site of the Fe-hydrogenase from Desulfovibrio desulfuricans. J. Am. Chem. Soc. 122, 11232 (2000)
Y. Nicolet, A.L. de Lacey, X. Vernède, V.M. Fernandez, E.C. Hatchikian, J.C. Fontecilla-Camps, Crystallographic and FTIR spectroscopic evidence of changes in Fe coordination upon reduction of the active site of the Fe-only hydrogenase from Desulfovibrio desulfuricans. J. Am. Chem. Soc. 123, 1596 (2001)
G. Berggren, A. Adamska, C. Lambertz, T.R. Simmons, J. Esselborn, M. Atta, S. Gambarelli, J.M. Mouesca, E. Reijerse, W. Lubitz, T. Happe, V. Artero, M. Fontecave, Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 499, 66 (2013)
J. Esselborn, C. Lambertz, A. Adamska-Venkatesh, T. Simmons, G. Berggren, J. Noth, J. Siebel, A. Hemschemeier, V. Artero, E. Reijerse, M. Fontecave, W. Lubitz, T. Happe, Spontaneous activation of [FeFe]-hydrogenases by an inorganic [2Fe] active site mimic. Nat. Chem. Biol. 9, 607 (2013)
C. Sommer, A. Adamska-Venkatesh, K. Pawlak, J.A. Birrell, O. Rüdiger, E.J. Reijerse, W. Lubitz, Proton coupled electronic rearrangement within the H-cluster as an essential step in the catalytic cycle of [FeFe] hydrogenases. J. Am. Chem. Soc. 139, 1440 (2017)
W. Lubitz, E. Reijerse, M. van Gastel, [NiFe] and [FeFe] hydrogenases studied by advanced magnetic resonance techniques. Chem. Rev. 107, 4331 (2007)
J.W. Peters, G.J. Schut, E.S. Boyd, D.W. Mulder, E.M. Shepard, J.B. Broderick, P.W. King, M.W.W. Adams, [FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation. Biochim. Biophys. Acta 1827, 1350 (2013)
J.H. Baricuatro, Y.G. Kim, F.H. Saadi, C.C.L. McCrory, J. Sanabria-Chinchilla, D. Crouthers, M.Y. Darensbourg, M.P. Soriaga, Heterogenization of a water-insoluble molecular complex for catalysis of the proton-reduction reaction in highly acidic aqueous solutions. Electrocatalysis 5, 226 (2014)
V.V. Khrizanforova, I.R. Knyazeva, V.I.M. Sokolova, I.R. Nizameev, T.V. Gryaznova, M.K. Kadirov, A.R. Burilov, O.G. Sinyashin, Y.H. Budnikova, Nickel complexes based on thiophosphorylated calix[4]resorcinols as effective catalysts for hydrogen evolution. Electrocatalysis 6, 357 (2015)
T.B. Rauchfuss, Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere. Acc. Chem. Res. 48, 2107 (2015)
J.A. Denny, M.Y. Darensbourg, Metallodithiolates as ligands in coordination, bioinorganic, and organometallic chemistry. Chem. Rev. 115, 5248 (2015)
V. Artero, G. Berggren, M. Atta, G. Caserta, S. Roy, L. Pecqueur, M. Fontecave, From enzyme maturation to synthetic chemistry: The case of hydrogenases. Acc. Chem. Res. 48, 2380 (2015)
C. Wombwell, C.A. Caputo, E. Reisner, [NiFeSe]-hydrogenase chemistry. Acc. Chem. Res. 48, 2858 (2015)
T. Xu, D.F. Chen, X.L. Hu, Hydrogen-activating models of hydrogenases. Coord. Chem. Rev. 303, 32 (2015)
S. Gao, J.L. Fan, S.G. Sun, F.L. Song, X.J. Peng, Q. Duan, D.Y. Jiang, Q.C. Liang, Di/mono-nuclear iron(I)/(II) complexes as functional models for the 2Fe2S subunit and distal Fe moiety of the active site of [FeFe] hydrogenases: Protonations, molecular structures and electrochemical properties. Dalton Trans. 41, 12064 (2012)
B.E. Barton, T.B. Rauchfuss, Terminal hydride in [FeFe]-hydrogenase model has lower potential for H2 production than the isomeric bridging hydride. Inorg. Chem. 47, 2261 (2008)
S. Tschierlei, S. Ott, R. Lomoth, Spectroscopically characterized intermediates of catalytic H2 formation by [FeFe] hydrogenase models, energy environ. Sci. 4, 2340 (2011)
R. Zaffaroni, T.B. Rauchfuss, D.L. Gray, L. De Gioia, G. Zampella, Terminal vs bridging hydrides of diiron dithiolates: Protonation of Fe2(dithiolate)(CO)2(PMe3)4. J. Am. Chem. Soc. 134, 19260 (2012)
L. Schwartz, P.S. Singh, L. Eriksson, R. Lomoth, S. Ott, Tuning the electronic properties of Fe2(μ-arenedithiolate)(CO)6–n(PMe3)n (n = 0, 2) complexes related to the [Fe–Fe]-hydrogenase active site. C. R. Chimie 11, 875 (2008)
S. Kaur-Ghumaan, L. Schwartz, R. Lomoth, M. Stein, S. Ott, Catalytic hydrogen evolution from mononuclear iron(II) carbonyl complexes as minimal functional models of the [FeFe] hydrogenase active site. Angew. Chem. Int. Ed. 49, 8033 (2010)
M. Beyler, S. Ezzaher, M. Karnahl, M.P. Santoni, R. Lomoth, S. Ott, Pentacoordinate iron complexes as functional models of the distal iron in [FeFe] hydrogenases. Chem. Commun. 47, 11662 (2011)
J.M. Gardner, M. Beyler, M. Karnahl, S. Tschierlei, S. Ott, L. Hammarström, Light-driven electron transfer between a photosensitizer and a proton-reducing catalyst co-adsorbed to NiO. J. Am. Chem. Soc. 134, 19322 (2012)
A. Orthaber, M. Karnahl, S. Tschierlei, D. Streich, M. Stein, S. Ott, Coordination and conformational isomers in mononuclear iron complexes with pertinence to the [FeFe] hydrogenase active site. Dalton Trans. 43, 4537 (2014)
S. Roy, S.K.S. Mazinani, T.L. Groy, L. Gan, P. Tarakeshwar, V. Mujica, A.K. Jones, Catalytic hydrogen evolution by Fe(II) carbonyls featuring a dithiolate and a chelating phosphine. Inorg. Chem. 53, 8919 (2014)
M. Natarajan, H. Faujdar, S.M. Mobin, M. Stein, S. Kaur-Ghumaan, A mononuclear iron carbonyl complex [Fe(μ-bdt)(CO)2(PTA)2] with bulky phosphine ligands: A model for the [FeFe] hydrogenase enzyme active site with an inverted redox potential. Dalton Trans. 46, 10050 (2017)
G.A.N. Felton, A.K. Vannucci, J. Chen, L.T. Lockett, N. Okumura, B.J. Petro, U.I. Zakai, D.H. Evans, R.S. Glass, D.L. Lichtenberger, Hydrogen generation from weak acids: Electrochemical and computational studies of a diiron hydrogenase mimic. J. Am. Chem. Soc. 129, 12521 (2007)
K. Izutsu, Acid-Base Dissociation Constants in Dipolar Aprotic Solvents, IUPAC Chemical Data Series No. 35 (Blackwell Scientific Publications, Oxford, 1990)
F. Gloaguen, J.D. Lawrence, M. Schmidt, S.R. Wilson, T.B. Rauchfuss, Synthetic and structural studies on [Fe2(SR)2(CN)x(CO)6–x]x– As active site models for Fe-only hydrogenases. J. Am. Chem. Soc. 123, 12518 (2001)
D. Streich, M. Karnahl, Y. Astuti, C.W. Cady, L. Hammarström, R. Lomoth, S. Ott, Comparing the reactivity of benzenedithiolate- versus alkyldithiolate-bridged Fe2(CO)6 complexes with competing ligands. Eur. J. Inorg. Chem. 2011, 1106 (2011)
S.J. George, Z. Cui, M. Razavet, C.J. Pickett, The di-iron subsite of all-iron hydrogenase: Mechanism of cyanation of a synthetic {2Fe3S}–carbonyl assembly. Chem. Eur. J. 8, 4037 (2002)
T.B. Rauchfuss, S.M. Contakes, S.C.N. Hsu, M.A. Reynolds, S.R. Wilson, The influence of cyanide on the carbonylation of iron(II): Synthesis of Fe–SR–CN–CO centers related to the hydrogenase active sites. J. Am. Chem. Soc. 123, 6933 (2001)
W.F. Liaw, N.H. Lee, C.H. Chen, C.M. Lee, G.H. Lee, S.M. Peng, Dinuclear and mononuclear iron(II)-thiolate complexes with mixed CO/CN− ligands: Synthetic advances for iron sites of [Fe]-only hydrogenases. J. Am. Chem. Soc. 122, 488 (2000)
J.F. Capon, F. Gloaguen, P. Schollhammer, J. Talarmin, Activation of proton by the two-electron reduction of a di-iron organometallic complex. J. Electroanal. Chem. 595, 47 (2006)
F. Gloaguen, J.D. Lawrence, T.B. Rauchfuss, Biomimetic hydrogen evolution catalyzed by an iron carbonyl thiolate. J. Am. Chem. Soc. 123, 9476 (2001)
R. Mejia-Rodriguez, D. Chong, J.H. Reibenspies, M.P. Soriaga, M.Y. Darensbourg, The hydrophilic phosphatriazaadamantane ligand in the development of H2 production electrocatalysts: Iron hydrogenase model complexes. J. Am. Chem. Soc. 126, 12004 (2004)
L.C. Song, Z.Y. Yang, H.Z. Bian, Y. Liu, H.T. Wang, X.F. Liu, Q.M. Hu, Diiron oxadithiolate type models for the active site of iron-only hydrogenases and biomimetic hydrogen evolution catalyzed by Fe2(μ-SCH2OCH2S-μ)(CO)6. Organometallics 24, 6126 (2005)
P. Li, M. Wang, C.J. He, G.H. Li, X.Y. Liu, C.N. Chen, B. Åkermark, L.C. Sun, Influence of tertiary phosphanes on the coordination configurations and electrochemical properties of iron hydrogenase model complexes: Crystal structures of [(μ-S2C3H6)Fe2(CO)6–nLn] (L = PMe2Ph, n = 1, 2; PPh3, P(OEt)3, n = 1). Eur. J. Inorg. Chem. 2005, 2506 (2005)
J. Sanabria-Chinchilla, A. Javier, D. Crouthers, J.H. Baricuatro, M.Y. Darensbourg, M.P. Soriaga, Immobilization-enabled proton reduction catalysis by a di-iron hydrogenase mimic. Electrocatalysis 5, 5 (2014)
P.S. Singh, H.C. Rudbeck, P. Huang, S. Ezzaher, L. Eriksson, M. Stein, S. Ott, R. Lomoth, (I,0) mixed-valence state of a diiron complex with pertinence to the [FeFe]-hydrogenase active site: An IR, EPR, and computational study. Inorg. Chem. 48, 10883 (2009)
I.A. de Carcer, A. DiPasquale, A.L. Rheingold, D.M. Heinekey, Active-site models for iron hydrogenases: Reduction chemistry of dinuclear iron complexes. Inorg. Chem. 45, 8000 (2006)
S.P. Best, S.J. Borg, J.M. White, M. Razavet, C.J. Pickett, On the structure of a proposed mixed-valent analogue of the diiron subsite of [FeFe]-hydrogenase. Chem. Commun. 42, 4348 (2007)
G.F. Qian, H.L. Wang, W. Zhong, X.M. Liu, Electrochemical investigation into the electron transfer mechanism of a diiron hexacarbonyl complex bearing a bridging naphthalene moiety. Electrochim. Acta 163, 190 (2015)
W. Zhong, Z.Y. Xiao, G.F. Qian, X.M. Liu, The influence of a peripheral functional group of diiron hexacarbonyl complexes on their electrochemistry and electrocatalytic reduction of proton. Electrochim. Acta 247, 779 (2017)
A.K. Vannucci, S. Wang, G.S. Nichol, D.L. Lichtenberger, D.H. Evans, R.S. Glass, Electronic and geometric effects of phosphatriazaadamantane ligands on the catalytic activity of an [FeFe] hydrogenase inspired complex. Dalton Trans. 39, 3050 (2010)
I.K. Pandey, S.M. Mobin, N. Deibel, B. Sarkar, S. Kaur-Ghumaan, Diiron benzenedithiolate complexes relevant to the [FeFe] hydrogenase active site. Eur. J. Inorg. Chem. 2015, 2875 (2015)
G.A.N. Felton, R.S. Glass, D.L. Lichtenberger, D.H. Evans, Iron-only hydrogenase mimics. Thermodynamic aspects of the use of electrochemistry to evaluate catalytic efficiency for hydrogen generation. Inorg. Chem. 45, 9181 (2006)
B.E. Barton, M.T. Olsen, T.B. Rauchfuss, Aza- and oxadithiolates are probable proton relays in functional models for the [FeFe]-hydrogenases. J. Am. Chem. Soc. 130, 16834 (2008)
Ö.F. Erdem, L. Schwartz, M. Stein, A. Silakov, S. Kaur-Ghumaan, P. Huang, S. Ott, E.J. Reijerse, W. Lubitz, A model of the [FeFe] hydrogenase active site with a biologically relevant azadithiolate bridge: A spectroscopic and theoretical investigation. Angew. Chem. Int. Ed. 50, 1439 (2011)
G.F. Qian, W. Zhong, Z.H. Wei, H.L. Wang, Z.Y. Xiao, L. Long, X.M. Liu, Diiron hexacarbonyl complexes bearing naphthalene-1,8-dithiolate bridge moiety as mimics of the sub-unit of [FeFe]-hydrogenase: Synthesis, characterisation and electrochemical investigations. New J. Chem. 39, 9752 (2015)
C. Tard, X.-M. Liu, D.L. Hughes, C.J. Pickett, A novel {FeI–FeII–FeII–FeI} iron thiolate carbonyl assembly which electrocatalyses hydrogen evolution. Chem. Commun. 1, 133 (2005)
Z.M. Li, X.H. Zeng, Z.G. Niu, X.M. Liu, Electrocatalytic investigations of a tri-iron cluster towards hydrogen evolution and relevance to [FeFe]-hydrogenase. Electrochim. Acta 54, 3638 (2009)
S.J. Borg, T. Behrsing, S.P. Best, M. Razavet, X.M. Liu, C.J. Pickett, Electron transfer at a dithiolate-bridged diiron assembly: Electrocatalytic hydrogen evolution. J. Am. Chem. Soc. 126, 16988 (2004)
S.J. Borg, S.K. Ibrahim, C.J. Pickett, S.P. Best, Electrocatalysis of hydrogen evolution by synthetic diiron units using weak acids as the proton source: Pathways of doubtful relevance to enzymic catalysis by the diiron subsite of [FeFe] hydrogenase. C. R. Chimie 11, 852 (2008)
S. Roy, J.A. Laureanti, T.L. Groy, A.K. Jones, Synthesis and electrocatalytic activity of [FeFe]-hydrogenase model complexes with non-innocent chelating nitrogen-donor ligands. Eur. J. Inorg. Chem. 2017, 2942 (2017)
F. Quentel, F. Gloaguen, Kinetic and thermodynamic aspects of the electrocatalysis of acid reduction in organic solvent using molecular diiron-dithiolate compounds. Electrochim. Acta 110, 641 (2013)
J.M. Savéant, Molecular catalysis of electrochemical reactions. Mechanistic aspects. Chem. Rev. 108, 2348 (2008)
M.L. Helm, M.P. Stewart, R.M. Bullock, M.R. DuBois, D.L. DuBois, A synthetic nickel electrocatalyst with a turnover frequency above 100,000 s−1 for H2 production. Science 333, 863 (2011)
A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, 2nd edn. (Wiley, New York, 2001)
G.A.N. Felton, C.A. Mebi, B.J. Petro, A.K. Vannucci, D.H. Evans, R.S. Glass, D.L. Lichtenberger, Review of electrochemical studies of complexes containing the Fe2S2 core characteristic of [FeFe]-hydrogenases including catalysis by these complexes of the reduction of acids to form dihydrogen. J. Organomet. Chem. 694, 2681 (2009)
I.K. Pandey, M. Natarajan, S. Kaur-Ghumaan, Hydrogen generation: Aromatic dithiolate-bridged metal carbonyl complexes as hydrogenase catalytic site. J. Inorg. Biochem. 143, 88 (2015)
Acknowledgments
We are grateful to the National Natural Science Foundation of China (no. 21201022, 61106050, 61774023), the Specialized Research Fund for the Doctoral Program of Higher Education (New Teachers, no. 20122216120001), the Scientific and Technological “13th Five-Year Plan” Project of Jilin Provincial Department of Education (no. JJKH20170609KJ), and the Scientific and Technological Development Project of Jilin Province (no. 20150311086YY) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, S., Liang, QC., Duan, Q. et al. Electrochemical Proton Reductions Catalyzed by the Simpler Hexacoordinate Iron Compounds as Functional Mimics of the Active Site in [FeFe] Hydrogenase. Electrocatalysis 9, 563–572 (2018). https://doi.org/10.1007/s12678-017-0453-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-017-0453-z