Abstract
Water electrolysis requires durability during a fluctuating power supply in power-to-gas application with renewable energies. The previously developed lithiated NiO modified Ni (Li x Ni2-x O2/Ni) has a high catalytic activity and durability during potential cycling, whereas the relatively high surface oxide resistance due to preparation of the oxidation at high temperature with a LiOH coating. In order to improve the catalytic activity, we proposed optimization of the oxide layer by thermal decomposition with various precursor coatings. The oxide layers prepared with acetate and nitrate precursors were dense and porous, respectively. The initial activity obtained from the acetate precursor electrode was higher than that of the nitrate precursor and the oxidation with the LiOH coating. Although the nitrate precursor electrode suffered from the same degradation as the Ni anode during potential cycling, the activity of the acetate precursor electrode as well as the oxidized electrode with the LiOH coating increased, and the activity for the former was almost the same as the initial Ni anode after 20,000 cycles. The lower preparation temperature of the acetate precursor would suppress the formation of the electron resistive nickel oxide between the base nickel and lithiated NiO, which was observed for the oxidized electrode with the LiOH coating as well. While the double-layer capacitance and redox peak around 1.3 V vs. RHE of the Ni and the nitrate precursor electrode significantly increased with Ni(IV) formation during potential cycling, those of the acetate precursor electrode slightly increased without any Ni(IV) formation. Therefore, the dense Li x Ni2-x O2 prepared with acetate has a good electric conductivity and catalytic activity with a high durability during potential cycling as the anode of an alkaline water electrolyzer for renewable power sources.

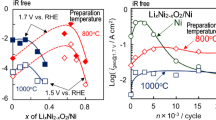
As a new oxygen evolution reaction (OER) electrocatalyst on a nickel substrate, thermal decomposition coating of lithiated nickel oxide with acetate precursor has a high OER current density with good durability under potential cycling, because the coating was more dense than the nitrate precursor coating, and thinner and had a lower resistance than the high temperature oxidation treatment with the LiOH melt.













Similar content being viewed by others
References
J. Divisek, H. Schmitz, A bipolar cell for advanced alkaline water. Int. J. Hydrog. Energy 7, 703–710 (1982)
D.E. Hall, Alkaline water electrolysis anode materials. J. Electrochem. Soc. 132, 41–48 (1985)
P. Rasiyah, A.C.C. Tseung, A mechanistic study of oxygen evolution on Li-doped Co3O4. J. Electrochem. Soc. 130, 365–368 (1983)
R.N. Singh, D. Mishra, Anindita, A.S.K. Sinha, A. Singh, Novel electrocatalysts for generating oxygen from alkaline water electrolysis. Electrochem. Commun. 9, 1369–1373 (2007)
R.N. Singh, S.K. Tiwari, S.P. Singh, A.N. Jain, N.K. Singh, Activity of high specific surface area perovskite-type LaNiO3, via sol-gel route for electrolytic oxygen evolution in alkaline solution. Int. J. Hydrog. Energy 22, 557–562 (1997)
R. Boggio, A. Carugati, S. Trasatti, Electrochemical surface properties of Co3O4 electrodes. J. Appl. Electrochem. 17, 828–840 (1987)
M. Hamdani, M.I.S. Pereira, J. Douch, A. Ait Addi, Y. Berghoute, M.H. Mendonça, Physicochemical and electrocatalytic properties of Li-Co3O4 anodes prepared by chemical spray pyrolysis for application in alkaline water electrolysis. Electrochim. Acta 49, 1555–1563 (2004)
M.E.G. Lyons, R.L. Doyle, I. Godwin, M. O’Brien, L. Russell, Hydrous nickel oxide: redox switching and the oxygen evolution reaction in aqueous alkaline solution. J. Electrochem. Soc. 159, H932–H944 (2012)
Y. Uchino, T. Kobayashi, S. Hasegawa, I. Nagashima, Y. Sunada, A. Manabe, Y. Nishiki, S. Mitsushima, Relationship between the redox reactions on a bipolar plate and reverse current after alkaline water electrolysis. Electrocatalysis (2017). https://doi.org/10.1007/s12678-017-0423-5
B.E. Conway, M.A. Sattar, D. Gilroy, Electrochemistry of the nickel-oxide electrode-v. self-passivation effects in oxygen-evolution kinetics. Electrochim. Acta 14, 677–694 (1969)
H. Ichikawa, K. Matsuzawa, Y. Kohno, I. Nagashima, Y. Sunada, Y. Nishiki, A. Manabe, S. Mitsushima, Durability and activity of modified nickel anode for alkaline water electrolysis. ECS Trans. 58, 9–15 (2014)
S. Fujita, I. Nagashima, Y. Sunada, Y. Nishiki, S. Mitsushima, Electrocatalytic activity and durability of Li x Ni2-x O2 electrode prepared by oxidation with LiOH melt for alkaline water electrolysis. Electrocatalysis 8, 422–429 (2017)
A. Manabe, M. Kashiwase, T. Hashimoto, T. Hayashida, A. Kato, K. Hirao, I. Shimomura, I. Nagashima, Basic study of alkaline water electrolysis. Electrochim. Acta 100, 249–256 (2013)
T. Oyama, Y. Iimura, K. Takeuchi, Synthesis of ( Cr x V1-x )2O3 fine particles by a laser-induced vapor-phase reaction and their crystal structure. J. Mater. Sci. 4, 439–444 (1999)
W. Li, J.N. Reimers, J.R. Dahn, Crystal structure of Li x Ni2-x O2 and a lattice gas model for the order-disorder transition. Phys. Rev. B 46, 3236–3247 (1992)
P.W.T. Lu, S. Srinivasan, Electrochemical-ellipsometric studies of oxide film formed on nickel during oxygen evolution. J. Electrochem. Soc. 125, 1416–1422 (1978)
M.C. Biesinger, B.P. Payne, L.W.M. Lau, A. Gerson, R.S.C. Smart, X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 41, 324–332 (2009)
Acknowledgements
The Institute of Advanced Sciences (IAS) in YNU is supported by the MEXT Program for Promoting the Reform of National Universities. We appreciate all persons involved in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, S., Nagashima, I., Nishiki, Y. et al. The Effect of Li x Ni2-x O2/Ni with Modification Method on Activity and Durability of Alkaline Water Electrolysis Anode. Electrocatalysis 9, 162–171 (2018). https://doi.org/10.1007/s12678-017-0439-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-017-0439-x