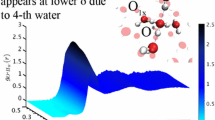
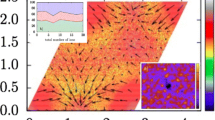
Abstract
Proton transfer processes in water are of fundamental importance for, among others, electrochemical proton discharge. Empirical valence bond (EVB) approaches were shown in the past to be a versatile tool for modeling complex phenomena such as proton discharge at metal electrodes. By replacing empirical fitting procedures with on-the-fly quantum chemistry (QC) calculations, we arrive at a transferable and systematically tunable description of proton transfer in water with EVB.

ᅟ







Similar content being viewed by others
References
R.A Marcus, J. Phys. Chem. 72, 891 (1968)
C.J.D. von Grotthus, Ann. Chim. LVIII, 54 (1806)
D. Marx, Chem. Phys. Chem. 7(9), 1848–1870 (2006). doi:10.1002/cphc.200600128
O. Pecina, W Schmickler, J. Electroanal. Chem. 431, 47–50 (1997)
O. Pecina, W Schmickler, Chem. Phys. 228, 265–277 (1998)
G.A Voth, Acc. Chem. Res. 39, 143–150 (2006). doi:10.1021/ar0402098
A. Warshel, R.M Weiss, J. Am. Chem. Soc. 102, 6218 (1980)
J. Lobaugh, G.A Voth, J. Chem. Phys. 104, 2056–2069 (1996)
D.E. Sagnella, M.E Tuckerman, J. Chem. Phys. 108, 2073–2083 (1997)
R. Vuilleumier, D. Borgis, J. Chem. Phys. 111, 4251–4266 (1999)
U.W. Schmitt, G.A. Voth, J. Chem. Phys. 111, 9361–9381 (1999)
U.W. Schmitt, G.A. Voth, J. Phys. Chem. B. 102, 5547–5551 (1998)
Y. Wu, H. Chen, F. Wang, F. Paesani, G.A Voth, J. Phys. Chem. B. 112, 467–482 (2008). doi:10.1021/jp076658h
S. Walbran, A.A Kornyshev, J. Chem. Phys. 114, 10039–10048 (2001)
E. Spohr, P. Commer, A.A Kornyshev, J. Phys. Chem. B. 106, 10560–10569 (2002)
S. Braun-Sand, A. Burykin, Z.T. Chu, A Warshel, J. Phys. Chem. B. 109, 583–592 (2005). doi:10.1021/jp0465783
F. Wilhelm, W. Schmickler, R.R. Nazmutdinov, E. Spohr, J. Phys. Chem. C. 112, 10814–10826 (2008)
F. Wilhelm, W. Schmickler, E. Spohr, J. Phys.: Condens. Matter. 22, 175001 (2010)
F. Wilhelm, W. Schmickler, R. Nazmutdinov, E. Spohr, Eletrochim. Acta. 56, 10632–10644 (2011)
H.M. Senn, W Thiel, Angew. Chem. Int. Ed. 48, 1198 (2009)
N. Bernstein, C. Varnai, I. Solt, S.A. Winfield, M.C. Payne, I. Simon, M. Fuxreiter, G Csanyi, Phys. Chem. Chem. Phys. 14, 646–656 (2012)
S. Dohm, E. Spohr, M. Korth, J. Comput. Chem. 38, 51 (2017)
J.P. Perdew, K. Burke, M Ernzerhof, Phys. Rev. Lett. 77(18), 3865 (1996)
F. Weigend, R Ahlrichs, Phys. Chem. Chem. Phys. 7, 3297 (2005)
S. Grimme, J. Comput. Chem. 27, 1787 (2006)
F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2(1), 73–78 (2012)
Acknowledgements
We gratefully acknowledge financial support by DFG within the framework of the DFG Research Unit 1376 “Elementary reaction steps in electrocatalysis: Theory meets Experiment”. ES is also grateful for support by the Cluster of Excellence RESOLV (EXC1069) funded by the Deutsche Forschungsgemeinschaft. MK and SD would like to thank the Barbara Mez-Starck Foundation for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dohm, S., Spohr, E. & Korth, M. Merging Empirical Valence Bond Theory with Quantum Chemistry to Model Proton Transfer Processes in Water. Electrocatalysis 8, 630–636 (2017). https://doi.org/10.1007/s12678-017-0396-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-017-0396-4