Abstract
Objectives
Mindfulness-Oriented Recovery Enhancement (MORE) is an integrative intervention designed to ameliorate addiction, chronic pain, and psychiatric symptoms. Although multiple randomized controlled trials (RCTs) have examined the clinical efficacy of MORE, no study has quantitatively synthesized this body of research. Thus, we conducted a meta-analysis of RCTs examining the effects of MORE on addictive behaviors, craving, opioid dose, pain, and psychiatric symptoms.
Methods
Relevant manuscripts were identified through comprehensive searches of four bibliographic databases. Two- and three-level random-effects models were used to generate synthesized effect size estimates, and meta-regressions were performed to examine whether study and sample characteristics influenced the magnitude of aggregate effect sizes.
Results
Our search identified 16 manuscripts reporting data from eight RCTs (N = 816). Moderate to small effects in favor of MORE were observed for addictive behaviors (SMC = − .54, p = .007), craving (SMC = − .42, p = .010), opioid dose (MC = − 17.95, p < .001), chronic pain (SMC = − .60, p < .001), and psychiatric symptoms (SMC = − .34, p < .001). MORE’s effects on psychiatric symptoms and craving were not moderated by participant race, gender, age, or income.
Conclusions
Study findings provide empirical evidence of MORE’s efficacy for a wide diversity of individuals, and as such, MORE should now be disseminated broadly throughout the healthcare system.
Meta-analysis Pre-registration: PROSPERO #CRD42022319006
Similar content being viewed by others
Approximately 275 million people worldwide use addictive substances each year, and of these, 36.3 million have a substance use disorder (SUD; UNODC, 2021). Since 1990, the global prevalence of drug and alcohol use has increased and is now the leading preventable cause of death worldwide (Murray et al., 2020; Ritchie & Roser, 2019). In the United States (US), over 91,000 Americans died from drug overdoses in 2020—the highest number ever recorded in a year (Hedegaard et al., 2021; Wilson, 2020). Millions more were impacted by the deleterious consequences of addiction on health, social well-being, and quality of life (Ignaszewski, 2021), which have only worsened under the coronavirus disease 2019 (COVID-19) pandemic (Czeisler et al., 2020). To curb this rapidly accelerating public health crisis, there is an urgent need for interventions that treat addiction and prevent overdose.
Nationally representative surveys estimate that 52.8% of individuals who experience an SUD in their lives will also experience chronic pain (Ilgen et al., 2010), and 37.9% will have a co-occurring psychiatric disorder (Han et al., 2017). Likewise, SUDs are not uncommon among individuals with psychiatric disorders, as well as among people with chronic pain—and particularly those patients prescribed long-term opioid therapy for analgesia (LTOT; Boscarino et al., 2010; Groenewald et al., 2018; Manchikanti et al., 2007; van Rijswijk et al., 2019; Vowles et al., 2015). When comorbid with SUDs, chronic pain and psychiatric disorders are associated with decreased functional impairment, a more chronic and protracted course of illness, and a higher risk of fatal overdose (Andersson et al., 2019; Ditre et al., 2019; Fernandez et al., 2019; Griffin et al., 2016; Jakubczyk et al., 2016; Larson et al., 2007; Morasco et al., 2011; Rogers et al., 2021; Sheu et al., 2008). Indeed, epidemiological research has linked increases in the prevalence of these intersecting “diseases of despair” to the declining health and rising mortality rates in the US (Case & Deaton, 2015, 2017; Glei & Preston, 2020; Woolf & Schoomaker, 2019).
Mounting evidence suggests that pain and mental health factors play a dynamic and reciprocal role in the initiation and maintenance of addictive behaviors. Psychoactive substances are often used to relieve physical pain and psychiatric distress (Khantzian, 1997). Such self-medication motives are commonly observed among chronic pain patients (Alford et al., 2016), as well as among individuals with mood and anxiety disorders (Turner et al., 2018). However, when substances are repeatedly used in the context of physical and/or emotional pain, these states may come to elicit craving that, in turn, motivates addictive behavior (Baker et al., 2004; Parisi et al., 2022b). As such, both interoceptive (e.g., physical or emotional pain) and exteroceptive cues (e.g., the sight of the drug) can activate the automatic habit of addiction even in the absence of the conscious intention to use drugs (Tiffany, 1990). Over time, addiction induces allostatic changes in the brain’s stress and reward systems that decrease sensitivity to natural reinforcing stimuli while increasing sensitivity to negative emotions and physical pain (Edwards et al., 2011; Koob, 2021; Shurman et al., 2010). As natural rewards lose their value and aversive experiences intensify, individuals may increase their substance consumption—whether illicit or prescribed—as a means of counteracting a progressively worsening emotional state. For individuals with chronic pain and/or psychiatric comorbidities, this allostatic shift may exacerbate the affective dysregulation, anhedonia, and blunting of reward function already associated with both conditions (Borsook et al., 2016; Elvemo et al., 2015; Koob, 2021; Manchikanti et al., 2007; Trøstheim et al., 2020), propelling a downward spiral of addictive behavior (Garland et al., 2013b).
To reverse this trajectory, interventions are needed to target the pathogenic mechanisms undergirding addiction, chronic pain, and psychiatric symptoms. To this end, Mindfulness-Oriented Recovery Enhancement (MORE) is an intervention grounded in affective neuroscience that unites mindfulness training, cognitive-behavioral therapy (CBT), and positive psychological principles into a transdiagnostic approach designed to simultaneously address addictive behavior, physical pain, and psychiatric symptoms.
MORE is a manualized, group-based intervention that provides sequenced training in mindfulness, reappraisal, and savoring skills (Garland, 2013). The original MORE protocol included 10 weekly sessions; subsequently, an 8-session protocol was developed and tested in multiple clinical trials. Participants first receive training in mindfulness meditation techniques to strengthen meta-awareness and cognitive control as a means of regulating maladaptive automatic habits and decreasing affective bias during appraisals of pain and craving sensations. This enhanced cognitive control facilitates subsequent training in reappraisal—a technique aimed at reinterpreting stressful life events to reduce negative emotions and reevaluating the adverse consequences of substance misuse. Finally, mindfulness amplifies the practice of savoring naturally rewarding experiences, a technique intended to boost reward processing, positive emotions, and meaning in life (Garland, 2013). Ultimately, the MORE treatment sequence culminates in a focus on self-transcendence—the sense of being connected to something greater than the self (Garland & Fredrickson, 2019; Hanley et al., 2018). Unlike other mindfulness-based interventions, MORE leverages principles from social-behavioral learning theory to enhance the motivation to practice mindfulness, build therapeutic expectancy, and positively reinforce success experiences to increase engagement with the intervention. Through an integration of mindfulness, reappraisal, and savoring techniques, MORE aims to restructure reward processing from valuation of drug rewards back to valuation of natural rewards as a means of decreasing addictive behaviors and craving (Garland, 2021). At the same time, MORE applies these techniques in an effort to reduce physical pain and psychiatric symptoms while enhancing well-being (Garland, 2016).
Since its inception, multiple clinical trials have supported the clinical efficacy of MORE across a diverse range of populations, including individuals with alcohol use disorders (AUDs; Garland et al., 2010); individuals receiving methadone-maintenance therapy (MMT) for opioid use disorders (OUD; Cooperman et al., 2021); chronic pain patients prescribed LTOT (Garland et al., 2014b, 2019b, 2022); individuals with co-occurring substance use and psychiatric disorders (Garland et al., 2016); and individuals with behavioral addictions (Li et al., 2017). A quantitative synthesis of this research is now needed to provide comprehensive evidence of MORE’s effects on addictive behaviors, craving, opioid dose, pain, and psychiatric symptoms, as well as to explore potential moderators of its efficacy. Given the underrepresentation of participants from diverse racial/ethnic groups and marginalized backgrounds in research examining mindfulness-based interventions for addiction (Spears, 2019), we were particularly interested in examining whether the impact of MORE on these outcomes was affected by the racial, gender, or socioeconomic composition of samples. Age was also identified as a salient moderating variable in light of research suggesting that older adults may have unique needs that can impact their responsiveness to SUD treatment (Choi et al., 2014; Kuerbis & Sacco, 2013). Therefore, the primary objectives of the present study were to (a) conduct a meta-analysis of randomized controlled trials examining the effects of MORE on addictive behaviors, craving, opioid dose, chronic pain, and psychiatric symptoms, and (b) examine whether the effects of MORE on clinical outcomes differed as a function of study and sample characteristics.
Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (Moher et al., 2009). A study protocol was submitted through PROSPERO, an international prospective register for review protocols (registration number, CRD42022319006).
Selection Criteria
Randomized controlled trials (RCTs) evaluating the effects of MORE on people with substance use disorders, substance use (including alcohol, nicotine, or prescription drugs including opioids), or behavioral addictions were eligible for inclusion. Studies were excluded if they did not evaluate MORE, used research designed other than RCTs (e.g., quasi-experimental, qualitative), did not include sufficient data to calculate an effect size, or were not published in peer-reviewed journals. No limitations were placed on studies based on the date of publication or language.
Search Strategy
A systematic, computerized search was conducted in the bibliographic databases Web of Science, PsychInfo, Scopus, and PubMed. Studies evaluating MORE were identified using the following search terms: Mindfulness-Oriented Recovery Enhancement OR Mindfulness Oriented Recovery Enhancement AND intervention OR program OR treatment. Searches were conducted in March 2022 and updated in July to identify any additional studies meeting inclusion criteria.
Following this initial search, two reviewers (A.P. and R.L.R.) worked independently to conduct a title and abstract review to assess articles’ eligibility for inclusion. Next, both reviewers read each study in full and excluded those that did not meet prespecified inclusion/exclusion criteria. There was near unanimity with respect to the studies identified as eligible during the title-and-abstract review. Disagreements during the full-text review were few and resolved through mutual discussion until consensus was reached. Additional relevant publications were identified by manually examining the reference lists of included articles and contacting the authors of studies eligible for inclusion.
Data Extraction
Two reviewers (A.P. and R.L.R.) independently extracted the following information from each manuscript: author, publication year, study aims, study setting, inclusion/exclusion criteria, sample size, mean age of participants, percentage of female participants, percentage of white participants, percentage of participants earning less than $25,000, intervention characteristics, length of treatment, primary outcomes, outcome measures, follow-up time points, and the means and standard deviations of primary outcomes at the longest follow-up time point available. If demographic information, means, or standard deviations were not reported in primary studies or supplementary materials, corresponding authors were contacted to request this data. When studies reported results based on analyses of ecological momentary assessments (EMA), means and standard deviations of EMAs collected during the first and last available week of measurement were computed for each time period and used for the meta-analysis. Following data extraction, both authors categorized extracted effect sizes into one of five outcomes: addiction-related behaviors, craving, opioid dose, pain, and psychiatric symptoms.
Risk of Bias
Risk of bias was assessed using Cochrane’s risk-of-bias tool for randomized trials (RoB 2; Sterne et al., 2019). Following the recommendation of the RoB 2, a code of “high risk”, “low risk”, or “some concerns” was assigned to the following five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was then used to rate the overall quality of evidence for each of our primary outcomes (Schünemann et al., 2008). Both assessments were conducted independently by two reviewers (A.P. and R.L.R.). Disagreements were rare and resolved through discussion.
Summary Measure
For addiction-related behaviors, craving, psychiatric symptoms, and chronic pain, standardized mean change (SMC) using raw score standardization was selected as our effect size metric. Here, the SMC between scores at pretreatment and the last available follow-up point was estimated for MORE and control conditions separately and multiplied by a bias correction factor. We selected the last available follow-up point as a conservative measure of the long-term impact of MORE. The final effect size estimate was computed by calculating the difference in the SMC between the two groups (Morris, 2008). To calculate the variance for SMC, the correlation between measurement points is required. Because this estimate was not reported by primary studies, we imputed a conservative value of r = 0.70 and re-estimated models using r values of 0.30, 0.50, and 0.90 to ensure the robustness of our findings (Rosenthal, 1984). No substantive differences emerged from these analyses.
All studies examining opioid dosing reported this outcome in morphine milligram equivalents (MME). Consequently, our effect size metric for opioid dose was the difference between unstandardized change scores for MORE and control conditions between pretreatment and the last available follow-up point. Measures of variation for mean change were estimated by converting F statistics and p values to standard errors and standard deviations (Higgins et al., 2019).
Effect Size Dependency
Many studies included in this review reported more than one effect size for each of our meta-analytic outcomes. However, including more than one effect size per study violates the assumption of independence that underlies traditional two-level random effects models (Borenstein et al., 2009). To address this dependency, we used three-level random effects models to evaluate outcomes in which studies contributed multiple effect sizes (pain, addictive behavior, psychiatric symptoms, craving), and a two-level model to evaluate opioid dose, as studies reporting on this outcome each contributed only one effect size. As with two-level random effects models, three-level models estimate sampling variance from individual effect sizes (level 1) and the variance between effect sizes from different studies (level 3). However, three-level models also yield an estimate of the variance between effect sizes drawn from the same study (level 2). This approach enables the extraction of multiple effect sizes in a non-aggregated form, thereby maximizing statistical power (Fernández-Castilla et al., 2020; Van den Noortgate et al., 2015; Van Den Noortgate & Onghena, 2003).
Data Analyses
Meta-analyses were performed using a multi-staged approach. We first calculated intercept-only two- and three-level random effects models, which yielded an estimate of the effect of MORE relative to comparison conditions. Next, we examined the heterogeneity of effect size estimates. For two-level random effects models, the I2 statistic and τ were used. For three-level models, heterogeneity was assessed by calculating three variance components: σ12, σ22, and the I2 statistic, which was partitioned across levels 2 and 3 to provide an estimate of the percentage of variance at each level of analysis (Cheung, 2019). For two-level models, I2 values above 25% were considered to reflect high levels of heterogeneity (Borenstein et al., 2009). For three-level models, independent log-likelihood ratio rests were conducted to test for heterogeneity at levels 2 and 3, and statistically significant tests were interpreted as evidence of heterogeneity (Assink & Wibbelink, 2016).
When significant heterogeneity was observed, we performed univariate random-effects meta-regressions to investigate study- and sample-level characteristics that may have impacted the effects of MORE on primary outcomes. Our moderating variables included the following: racial/ethnic composition (percentage of white participants), sample age, sample low-income socioeconomic status (percentage of the sample reporting an annual income of less than $25,000), gender composition (percentage of female participants), and year of publication. If studies reported income as a categorical variable, we used the closest available value when thresholds other than $25,000 were reported. As recommended by Fu et al. (2011) meta-regressions were only performed when six or more studies provided effect size estimates.
Publication bias was assessed through visual inspection of the symmetry of contour-enhanced funnel plots and Egger’s tests, which were modified for use in three-level models by estimating the variance component of each outcome as a model covariate. All models were estimated using the Restricted Maximum-Likelihood (REML) estimator (Pastor & Lazowski, 2018) using the “rma” and “rma.mv” functions of the metafor package (Viechtbauer, 2010) in RStudio (2021).
Results
Characteristics of Selected Studies
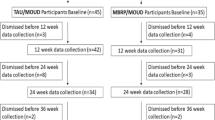
We screened 122 citations and 30 full-text articles. Sixteen manuscripts reporting data from eight RCTs of MORE (total N = 816) were ultimately included (see Fig. 1). Seven manuscripts reported primary outcomes from clinical trials (Cooperman et al., 2021; Garland et al., 2010, 2014b, 2016, 2019b, 2022; Li et al., 2017), seven were secondary analyses (Garland et al., 2014a, 2017b, 2019a, 2020; Hanley & Garland, 2020; Parisi et al., 2022a; Roberts et al., 2022), and two reported data from the same sample of participants taking part in a randomized controlled mechanistic study (Garland et al., 2021; Hudak et al., 2021). All studies were published in English between 2010 and 2022. Key characteristics of included RCTs and their corresponding manuscripts are detailed in Table 1.
Interventions
Across all RCTs, MORE was delivered as a manualized, group-based intervention consisting of weekly, 2-h sessions. Two RCTs examined 10-session versions of MORE developed for individuals with AUDs (Garland et al., 2010) and SUDs (Garland et al., 2016). The remaining RCTs examined 8-session versions of MORE developed to address opioid use among individuals with chronic pain (Garland et al., 2014b, 2019b, 2021, 2022) and internet gaming disorder (Li et al., 2017).
The majority of RCTs (75%) compared MORE to supportive psychotherapy groups (SG) matched to MORE in terms of their structure, intensity, and homework requirements (Garland et al., 2010, 2014b, 2019b, 2021; 2022; Li et al., 2017). One RCT compared MORE to methadone treatment-as-usual (TAU; Cooperman et al., 2021), and another RCT employed two comparison conditions: cognitive-behavioral therapy (CBT) and TAU (Garland et al., 2016). Means and standard deviations from the CBT condition were used to generate effect sizes for this latter study, and models were re-estimated using the TAU condition as a sensitivity analysis. No substantive differences were found. Intervention fidelity (e.g., therapist adherence and competence) for MORE and active comparison conditions (SG, CBT) was monitored in all but one RCT (Garland et al., 2010), with no major deviations reported.
Participants
The eight RCTs included in this meta-analysis examined 816 adult participants. Sample sizes ranged from 30 to 250, and the mean age of study samples ranged from 25 to 59. Though the majority of participants were male (58.1%) and white (67.5%), a substantial proportion of participants were female or from diverse and underrepresented racial/ethnic groups. Four RCTs enrolled individuals prescribed LTOT for chronic pain. Of these, one included individuals who evidenced opioid misuse (Garland et al., 2022), one included individuals who did not evidence opioid misuse at the time of study enrollment (Garland et al., 2019b), and two did not restrict participants by opioid misuse status (Garland et al., 2014b, 2021). The remaining studies recruited individuals with AUD (Garland et al., 2010); comorbid substance use and psychiatric disorders (Garland et al., 2016); internet gaming disorder (Li et al., 2017); and individuals with OUD and chronic pain receiving methadone treatment (Cooperman et al., 2021).
Methodological Characteristics
Figure 2 illustrates the methodological attributes of the eight included RCTs as assessed using the RoB 2. All trials reported the use of appropriate random sequence generation methods; likewise, intervention adherence was high across RCTs and supported by assessments of treatment fidelity. Most RCTs reported that assessors were blinded to treatment condition; however, as is common in psychosocial interventions, participants were not blinded in any study. Although several RCTs reported high levels of attrition, the quantity of missing data was comparable to other studies of psychosocial interventions for SUD (Lappan et al., 2020). Moreover, most studies (n = 7) performed intent-to-treat analyses with statistical techniques designed to account for missing data (e.g., maximum likelihood estimation of missing data). Standardized, validated measures were used to assess outcomes across studies. Although many study protocols (n = 6) were preregistered on clinicaltrials.gov, two studies were completed prior to when preregistration requirements became commonplace. As such, two trials were not registered, preventing us from ruling out the potential for biases in the selection of reported results for those trials. Details of the RoB 2 assessment are available in Online Resource 1.
Outcomes
The 16 included manuscripts produced 47 effect sizes reflecting the effects of MORE on addictive behavior, pain, psychiatric symptoms, craving, and opioid dose relative to comparison conditions. Results from all meta-analyses are reported in Table 2, and our summary of evidence is presented in Table 3.
Addictive Behaviors
Five RCTs (Fig. 3) produced six effect sizes on addictive behaviors, including opioid misuse (Garland et al., 2014b, 2019b, 2022), illicit drug use (Cooperman et al., 2021), and internet gaming disorder symptoms (Li et al., 2017). A statistically significant, moderate effect size was observed, suggesting that MORE participants experienced larger reductions in addictive behaviors than participants in comparison conditions (SMC = − 0.54, 95% CI [− 0.86, − 0.23], p = 0.007).
Craving
Seven RCTs (Fig. 4) reported ten effect sizes related to opioid (Cooperman et al., 2021; Hanley & Garland, 2020; Garland et al., 2014a, 2014b, 2019a; Parisi et al., 2022a), alcohol (Garland et al., 2010), video game (Li et al., 2017), and general substance craving (Garland et al., 2016). Follow-up points ranged from the last treatment week to 9 months posttreatment. A statistically significant, small-moderate effect size was observed favoring MORE (SMC = − 0.42, 95% CI [− 0.73, − 0.11], p = 0.014), such that MORE participants demonstrated larger reductions in craving than participants in comparison conditions.
Opioid Dose
Three RCTs produced three effect sizes related to opioid dosing among chronic pain patients prescribed LTOT (Fig. 5). Follow-up points ranged from 3 to 9 months posttreatment. Results revealed a statistically significant effect in favor of MORE, such that MORE participants evidenced a greater decrease in MME (MC = − 0.17.95 mg) relative to comparison conditions (95% CI [− 26.17, − 9.72], p < 0.001).
Chronic Pain
Four RCTs (Fig. 6) produced 10 effect sizes examining the effects of MORE on chronic pain-related outcomes, including pain severity (Garland et al., 2014b, 2019b, 2022), interference (Garland et al., 2014b, 2022), unpleasantness (Garland et al., 2019a), and intensity (Garland et al., 2019a). Follow-up points ranged from the last week of treatment to 9 months posttreatment. Overall, MORE was associated with statistically significant, moderate effect size decreases in pain relative to comparison conditions (SMC = − 0.60, 95% CI [− 0.83, − 0.37], p < 0.001).
Psychiatric Symptoms
Eight RCTs (Fig. 7) reported 18 effect sizes investigating the effects of MORE on a range of psychiatric symptoms, including general symptomatology (Garland et al., 2010), stress (Garland et al., 2010, 2014b, 2019a), distress (Garland et al., 2017b, 2022; Li et al., 2017; Roberts et al., 2022), well-being (Cooperman et al., 2021), depression (Garland et al., 2016, 2022), anxiety (Garland et al., 2016), and posttraumatic stress disorder symptoms (Garland et al., 2016, 2022). Follow-up points ranged from posttreatment to 9 months posttreatment. MORE was associated with statistically significant, small effect size reductions in psychiatric symptoms relative to comparison conditions (SMC = − 0.34, 95% CI [− 0.51, − 0.17], p < 0.001).
Publication Bias
Visual inspections of contour-enhanced funnel plots showed moderate levels of asymmetry for the effects of MORE on addictive behaviors, pain, and psychiatric symptoms (see Online Resource 2). Moreover, Egger’s regression tests were significant for all three outcomes, suggesting that publication bias or other sources of heterogeneity may have influenced the synthesized effect sizes (Table 2). Consequently, we re-analyzed these outcomes using trim-and-fill methods that account for the asymmetric distribution of studies around an omnibus effect. Nearly identical models were observed for addictive behaviors (SMC = − 0.49, p < 0.001, 95% CI [− 0.70, − 0.28]), and pain (SMC = − 0.64, p < 0.001, 95% CI [− 0.83, − 0.44]), while smaller yet significant effects were found for psychiatric symptoms (SMC = − 0.22, p = < 0.001, 95% CI [− 0.34, − 0.10]).
Moderation Analyses
Significant log-likelihood estimates were observed for craving, indicating heterogeneity of effect sizes. Moreover, an inspection of forest plots revealed several non-overlapping confidence intervals for pain and psychiatric symptoms, suggesting that both outcomes also had high levels of study heterogeneity. We therefore examined sample race/ethnicity, sample age, sample socioeconomic status, sample gender, and the year of study publication as moderating variables for psychiatric symptoms and craving, as an insufficient number of studies were available to conduct meta-regressions for pain-related outcomes (Lipsey, 2003). No significant moderating variables emerged from these analyses (see Table 4), suggesting that the effect of MORE on both outcomes was robust to the year of publication, as well as the demographic characteristics of study samples.
Discussion
This meta-analysis quantitatively synthesized the therapeutic effects of MORE. Sixteen manuscripts reporting outcomes from eight RCTs were included, which examined 816 participants with a broad array of addictive disorders, psychiatric symptoms, and chronic pain conditions. Our findings demonstrate that MORE produced significantly larger improvements in addictive behaviors, craving, opioid dosing, chronic pain, and psychiatric symptoms than a range of active comparison conditions. The majority of trials reviewed focused on people with chronic pain at risk for opioid misuse or OUD; results from the present study demonstrate that MORE is clearly an efficacious treatment for this group of patients—a growing population for whom effective interventions are lacking. Moreover, despite the diversity of included participants, MORE’s therapeutic effects on craving and psychiatric symptoms did not systematically differ as a function of participant age, race, gender, or income, suggesting that MORE may be efficacious for a wide diversity of individuals.
It is possible that MORE’s broad spectrum effects stem from its unique integration of mindfulness, reappraisal, and savoring practices. Unlike other MBIs, in MORE formal mindfulness practices are used to synergize later training in reappraisal and savoring skills, providing participants with a range of regulatory strategies that may be flexibly employed to target the manifold mechanisms implicated in addiction, psychiatric disorders, and chronic pain. For example, mindfulness may facilitate meta-awareness of pain, negative emotions, and drug cue-reactivity, enabling individuals to recognize and disrupt automatic attentional biases (Garland & Howard, 2013; Garland et al., 2017a), and habitual behavioral responses that drive addictive behaviors (Garland et al., 2013b; Tiffany, 1990). The metacognitive stance afforded by mindfulness training may broaden awareness to encompass previously unnoticed contextual information that accommodates cognitive reappraisal of stressful life circumstances (Garland et al., 2015; Goldin et al., 2021)—an emotion regulatory process that has been linked to lower levels of craving, psychiatric distress, and substance misuse (Dryman & Heimberg, 2018; Hudak et al., 2022; Kober et al., 2010; Roberts et al., 2022). In a complementary fashion, the attentional capacities strengthened by mindfulness training may be leveraged in the service of savoring to (a) remediate dysregulated reward function by amplifying positive affective and neurophysiological responses to naturally rewarding, salutary objects and events (Garland et al., 2014a, 2019b, 2021) and (b) enhancing the motivational drive to sustain adaptive behavioral changes (Garland, 2016). As cited above, though a systematic mechanistic research program has obtained evidence of the effects of MORE on the aforementioned processes, multivariate mediational models are now needed to determine the independent and interactive causal effects of each of these components on MORE’s clinical outcomes as revealed by the present meta-analysis.
Limitations and Future Research
Any conclusions or implications drawn from our findings should be tempered by the limitations of this meta-analysis. First, although we employed a multilevel analytic approach to maximize statistical power, the modest number of RCTs reviewed may have negatively impacted the reliability of effect size estimates. Additionally, half of the RCTs were stage 1 clinical trials with small samples and therefore may have been underpowered.
Second, we found evidence of heterogeneity for psychiatric symptoms, craving, and pain-related outcomes. Although random effects meta-regressions were performed, no moderating variable was found to be significant. Moreover, the few studies reporting pain-related outcomes precluded examination of effect size moderators. Consequently, the source of within- and between-study heterogeneity for all three outcomes remains unclear.
Third, an inspection of funnel plots and results from Egger’s regressions revealed moderate levels of asymmetry for addictive behaviors, pain, and psychiatric symptoms, indicating that results for these outcomes may have been subject to publication bias. Although trim-and-fill analyses suggested that such bias, if present, had little impact on our primary findings, these tests may have been underpowered and should thus be interpreted in light of this limitation. That said, according to our review of clinicaltrials.gov, no trials of MORE for addictive behaviors meeting our inclusion criteria were omitted from this meta-analysis.
Fourth, because the majority of RCTs assessed outcomes at posttreatment or 3 months posttreatment, additional research is needed to ascertain the long-term impact of MORE. Fifth, the majority of studies examining the effects of MORE on addictive behaviors focused on opioid misuse among individuals with chronic pain conditions, limiting the generalizability of findings. In that regard, we derived our effect size estimate of MORE for opioid misuse from a continuous measure of opioid misuse, the Current Opioid Misuse Measure (Butler et al., 2007). Use of this measure may underestimate MORE’s actual effect on opioid misuse; in the largest clinical trial of MORE to date (N = 250; Garland et al., 2022), MORE reduced a composite, binary index opioid misuse (triangulating self-report, blinded clinical interview, and drug urine screen) by 45% at 9-month follow-up, nearly tripling the effect of the supportive psychotherapy control condition (OR = 2.94 at 9 months).
Finally, this meta-analysis may be limited by bias in that it involved authors (e.g., E.L.G., A.W.H.) of many of the primary trials reviewed. To mitigate such bias, study identification, data extraction, coding, and analysis were conducted by authors (A.P., R.L.R.) who were not involved in the conduct of any of the trials reviewed, and neither E.L.G. nor A.W.H. performed the aforementioned processes. It should also be noted that the developer of MORE (E.L.G.) was a coauthor on publications from all the trials reviewed in this meta-analysis, though two of the trials were conducted by independent principal investigators (Cooperman et al., 2021; Li et al., 2017). Given that MORE is a young therapy, the involvement of the developer in these initial trials is perhaps unsurprising. However, as the evidence base on MORE continues to grow, independent teams of investigators should evaluate MORE in future trials.
To advance the growing empirical foundation of MORE, we propose several directions for future research. Multi-site large-scale RCTs with longer follow-up periods are needed to support the strength and sustainability of outcomes reported in this review. To establish the efficacy of MORE for addictive behaviors, future RCTs should also examine its effects among non-pain populations and obtain quantitative estimates of substance use and other addictive behaviors. Although several studies in this meta-analysis tested MORE among low-income racial and ethnic minority populations, and the overall non-white proportion of participants in these trials is 32.5%, recruiting additional participants from diverse racial, ethnic, and socioeconomic backgrounds remains an ongoing research priority. Such research could strengthen the generalizability of our findings and provide the statistical power needed to support more granular investigations regarding the populations for whom MORE may work most optimally. Finally, the majority of RCTs in this meta-analysis compared the effects of MORE to active comparison conditions, providing evidence that our findings cannot be explained by non-specific therapeutic factors. However, MORE is a multimodal intervention that targets a number of interconnected and complex mechanisms designed to maximize its clinical effects among individuals with addictive disorders, chronic pain, and psychiatric comorbidities. Dismantling trials are now needed to determine to what extent the mindfulness, reappraisal, and savoring components in MORE contribute to its therapeutic benefits. With continued effectiveness and implementation research, MORE should advance towards more widespread dissemination in healthcare.
References
References marked with an * are included in the meta-analysis
Alford, D. P., German, J. S., Samet, J. H., Cheng, D. M., Lloyd-Travaglini, C. A., & Saitz, R. (2016). Primary care patients with drug use report chronic pain and self-medicate with alcohol and other drugs. Journal of General Internal Medicine, 31(5), 486–491. https://doi.org/10.1007/s11606-016-3586-5
Andersson, H. W., Wenaas, M., & Nordfjærn, T. (2019). Relapse after inpatient substance use treatment: A prospective cohort study among users of illicit substances. Addictive Behaviors, 90, 222–228. https://doi.org/10.1016/j.addbeh.2018.11.008
Assink, M., & Wibbelink, C. J. M. (2016). Fitting three-level meta-analytic models in R: A step-by-step tutorial. The Quantitative Methods for Psychology, 12(3), 154–174. https://doi.org/10.20982/tqmp.12.3.p154
Baker, T. B., Piper, M. E., McCarthy, D. E., Majeskie, M. R., & Fiore, M. C. (2004). Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review, 111(1), 33–51. https://doi.org/10.1037/0033-295X.111.1.33
Borenstein, M., Hedges, L. V., Higgins, J., & Rothstein, H. R. (2009). Introduction to meta-analysis. John Wiley & Sons, Ltd.
Borsook, D., Linnman, C., Faria, V., Strassman, A. M., Becerra, L., & Elman, I. (2016). Reward deficiency and anti-reward in pain chronification. Neuroscience & Biobehavioral Reviews, 68, 282–297. https://doi.org/10.1016/j.neubiorev.2016.05.033
Boscarino, J. A., Rukstalis, M., Hoffman, S. N., Han, J. J., Erlich, P. M., Gerhard, G. S., & Stewart, W. F. (2010). Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction, 105(10), 1776–1782. https://doi.org/10.1111/j.1360-0443.2010.03052.x
Butler, S. F., Budman, S. H., Fernandez, K. C., Houle, B., Benoit, C., Katz, N., & Jamison, R. N. (2007). Development and validation of the Current Opioid Misuse Measure. Pain, 130, 144–156.
Case, A., & Deaton, A. (2015). Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences, 112(49), 15078–15083.
Case, A., & Deaton, A. (2017). Mortality and morbidity in the 21st century. Brookings Papers on Economic Activity, 2017(1), 397–476. https://doi.org/10.1353/eca.2017.0005
Cheung, M.W.-L. (2019). A Guide to conducting a meta-analysis with non-independent effect sizes. Neuropsychology Review, 29(4), 387–396. https://doi.org/10.1007/s11065-019-09415-6
Choi, N. G., DiNitto, D. M., & Marti, C. N. (2014). Treatment use, perceived need, and barriers to seeking treatment for substance abuse and mental health problems among older adults compared to younger adults. Drug and Alcohol Dependence, 145, 113–120. https://doi.org/10.1016/j.drugalcdep.2014.10.004
*Cooperman, N. A., Hanley, A. W., Kline, A., & Garland, E. L. (2021). A pilot randomized clinical trial of mindfulness-oriented recovery enhancement as an adjunct to methadone treatment for people with opioid use disorder and chronic pain: Impact on illicit drug use, health, and well-being. Journal of Substance Abuse Treatment, 127, 108468. https://doi.org/10.1016/j.jsat.2021.108468
Czeisler, M. É., Lane, R. I., Petrosky, E., Wiley, J. F., Christensen, A., Njai, R., Weaver, M. D., Robbins, R., Facer-Childs, E. R., Barger, L. K., Czeisler, C. A., Howard, M. E., & Rajaratnam, S. M. W. (2020). Mental Health, substance use, and suicidal ideation during the COVID-19 pandemic—United States, June 24–30, 2020. Morbidity and Mortality Weekly Report, 69(32), 1049–1057. https://doi.org/10.15585/mmwr.mm6932a1
Ditre, J. W., Zale, E. L., & LaRowe, L. R. (2019). A reciprocal model of pain and substance use: Transdiagnostic considerations, clinical implications, and future directions. Annual Review of Clinical Psychology, 15(1), 503–528. https://doi.org/10.1146/annurev-clinpsy-050718-095440
Dryman, M. T., & Heimberg, R. G. (2018). Emotion regulation in social anxiety and depression: A systematic review of expressive suppression and cognitive reappraisal. Clinical Psychology Review, 65, 17–42. https://doi.org/10.1016/j.cpr.2018.07.004
Edwards, R. R., Wasan, A. D., Michna, E., Greenbaum, S., Ross, E., & Jamison, R. N. (2011). Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. The Journal of Pain, 12(9), 953–963.
Elvemo, N. A., Landrø, N. I., Borchgrevink, P. C., & Håberg, A. K. (2015). Reward responsiveness in patients with chronic pain. European Journal of Pain, 19(10), 1537–1543. https://doi.org/10.1002/ejp.687
Fernandez, A. C., Bush, C., Bonar, E. E., Blow, F. C., Walton, M. A., & Bohnert, A. A. S. B. (2019). Alcohol and drug overdose and the influence of pain conditions in an addiction treatment sample. Journal of Addiction Medicine, 13(1), 61–68. https://doi.org/10.1097/ADM.0000000000000451
Fernández-Castilla, B., Jamshidi, L., Declercq, L., Beretvas, S. N., Onghena, P., & Van den Noortgate, W. (2020). The application of meta-analytic (multi-level) models with multiple random effects: A systematic review. Behavior Research Methods, 52(5), 2031–2052. https://doi.org/10.3758/s13428-020-01373-9
Fu, R., Gartlehner, G., Grant, M., Shamliyan, T., Sedrakyan, A., Wilt, T. J., Griffith, L., Oremus, M., Raina, P., Ismaila, A., Santaguida, P., Lau, J., & Trikalinos, T. A. (2011). Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. Journal of Clinical Epidemiology, 64(11), 1187–1197. https://doi.org/10.1016/j.jclinepi.2010.08.010
Garland, E. L. (2013). Mindfulness-Oriented Recovery Enhancement for addiction, stress, and pain. NASW Press.
Garland, E. L. (2016). Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: Novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Annals of the New York Academy of Sciences, 1373(1), 25–37. https://doi.org/10.1111/nyas.13034
Garland, E. L. (2021). Mindful positive emotion regulation as a treatment for addiction: From hedonic pleasure to self-transcendent meaning. Current Opinion in Behavioral Sciences, 39, 168–177.
Garland, E. L., Baker, A. K., & Howard, M. O. (2017a). Mindfulness-oriented recovery enhancement reduces opioid attentional bias among prescription opioid-treated chronic pain patients. Journal of the Society for Social Work and Research, 8(4), 493–509.
*Garland, E. L., Bryan, C. J., Finan, P. H., Thomas, E. A., Priddy, S. E., Riquino, M. R., & Howard, M. O. (2017b). Pain, hedonic regulation, and opioid misuse: Modulation of momentary experience by Mindfulness-Oriented Recovery Enhancement in opioid-treated chronic pain patients. Drug and Alcohol Dependence, 173, S65–S72. https://doi.org/10.1016/j.drugalcdep.2016.07.033
Garland, E. L., Farb, N. A., Goldin, P. R., & Fredrickson, B. L. (2015). Mindfulness broadens awareness and builds eudaimonic meaning: A process model of mindful positive emotion regulation. Psychological Inquiry, 26(4), 293–314. https://doi.org/10.1080/1047840X.2015.1064294
*Garland, E. L., Fix, S. T., Hudak, J. P., Bernat, E. M., Nakamura, Y., Hanley, A. W., Donaldson, G. W., Marchand, W. R., & Froeliger, B. (2021). Mindfulness-Oriented Recovery Enhancement remediates anhedonia in chronic opioid use by enhancing neurophysiological responses during savoring of natural rewards. Psychological Medicine, 1–10. https://doi.org/10.1017/S0033291721003834
Garland, E. L., & Fredrickson, B. L. (2019). Positive psychological states in the arc from mindfulness to self-transcendence: Extensions of the Mindfulness-to-Meaning Theory and applications to addiction and chronic pain treatment. Current Opinion in Psychology, 28, 184–191. https://doi.org/10.1016/j.copsyc.2019.01.004
Garland, E. L., Froeliger, B., & Howard, M. O. (2013a). Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Name Frontiers in Psychiatry, 4, 173.
*Garland, E. L., Froeliger, B., & Howard, M. O. (2014a). Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (berl), 231(16), 3229–3238. https://doi.org/10.1007/s00213-014-3504-7
Garland, E. L., Froeliger, B., Zeidan, F., Partin, K., & Howard, M. O. (2013b). The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience & Biobehavioral Reviews, 37(10), 2597–2607.
*Garland, E. L., Gaylord, S. A., Boettiger, C. A., & Howard, M. O. (2010). Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results of a randomized controlled pilot trial. Journal of Psychoactive Drugs, 42(2), 177–192
*Garland, E. L., Hanley, A. W., Kline, A., & Cooperman, N. A. (2019a). Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessments from a stage 1 randomized controlled trial. Drug and Alcohol Dependence, 203, 61–65. https://doi.org/10.1016/j.drugalcdep.2019a.07.007
*Garland, E. L., Hanley, A. W., Nakamura, Y., Barrett, J. W., Baker, A. K., Reese, S. E., Riquino, M. R., Froeliger, B., & Donaldson, G. W. (2022). Mindfulness-Oriented Recovery Enhancement vs supportive group therapy for co-occurring opioid misuse and chronic pain in primary care: A randomized clinical trial. JAMA Internal Medicine. Scopus. https://doi.org/10.1001/jamainternmed.2022.0033
*Garland, E. L., Hanley, A. W., Riquino, M. R., Reese, S. E., Baker, A. K., Salas, K., Yack, B. P., Bedford, C. E., Bryan, M. A., Atchley, R., Nakamura, Y., Froeliger, B., & Howard, M. O. (2019b). Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 87(10), 927–940. https://doi.org/10.1037/ccp0000390
Garland, E. L., & Howard, M. O. (2013). Mindfulness-oriented recovery enhancement reduces pain attentional bias in chronic pain patients. Psychotherapy and Psychosomatics, 82(5), 311–318.
*Garland, E. L., Hudak, J., Hanley, A. W., & Nakamura, Y. (2020). Mindfulness-oriented recovery enhancement reduces opioid dose in primary care by strengthening autonomic regulation during meditation. American Psychologist, 75(6), 840–852https://doi.org/10.1037/amp0000638
*Garland, E. L., Manusov, E. G., Froeliger, B., Kelly, A., Williams, J. M., & Howard, M. O. (2014b). Mindfulness-Oriented Recovery Enhancement for chronic pain and prescription opioid misuse: Results from an early stage randomized controlled trial. Journal of Consulting and Clinical Psychology, 82(3), 448–459. https://doi.org/10.1037/a0035798
*Garland, E. L., Roberts-Lewis, A., Tronnier, C. D., Graves, R., & Kelley, K. (2016). Mindfulness-Oriented Recovery Enhancement versus CBT for co-occurring substance dependence, traumatic stress, and psychiatric disorders: Proximal outcomes from a pragmatic randomized trial. Behaviour Research and Therapy, 77, 7–16https://doi.org/10.1016/j.brat.2015.11.012
Glei, D. A., & Preston, S. H. (2020). Estimating the impact of drug use on US mortality, 1999–2016. PLoS ONE, 15(1), e0226732. https://doi.org/10.1371/journal.pone.0226732
Goldin, P. R., Thurston, M., Allende, S., Moodie, C., Dixon, M. L., Heimberg, R. G., & Gross, J. J. (2021). Evaluation of cognitive behavioral therapy vs mindfulness meditation in brain changes during reappraisal and acceptance among patients with social anxiety disorder: A randomized clinical trial. JAMA Psychiatry. https://doi.org/10.1001/jamapsychiatry.2021.1862
Griffin, M. L., McDermott, K. A., McHugh, R. K., Fitzmaurice, G. M., Jamison, R. N., & Weiss, R. D. (2016). Longitudinal association between pain severity and subsequent opioid use in prescription opioid dependent patients with chronic pain. Drug and Alcohol Dependence, 163, 216–221.
Groenewald, C. B., Law, E. F., Fisher, E., Beals-Erickson, S. E., & Palermo, T. M. (2018). Associations between adolescent chronic pain and prescription opioid misuse in adulthood. The Journal of Pain.
Han, B., Compton, W. M., Blanco, C., & Colpe, L. J. (2017). Prevalence, treatment, and unmet treatment needs of us adults with mental health and substance use disorders. Health Affairs, 36(10), 1739–1747. https://doi.org/10.1377/hlthaff.2017.0584
*Hanley, A. W., & Garland, E. L. (2020). Salivary measurement and mindfulness-based modulation of prescription opioid cue-reactivity. Drug and Alcohol Dependence, 217, 108351 https://doi.org/10.1016/j.drugalcdep.2020.108351
Hanley, A. W., Nakamura, Y., & Garland, E. L. (2018). The Nondual Awareness Dimensional Assessment (NADA): New tools to assess nondual traits and states of consciousness occurring within and beyond the context of meditation. Psychological Assessment, 30(12), 1625.
Hedegaard, H., Miniño, A., Spencer, M. R., & Warner, M. (2021). Drug overdose deaths in the United States, 1999–2020. National Center for Health Statistics ( U.S.). https://doi.org/10.15620/cdc:112340
Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., & Welch, V. (2019). Cochrane handbook for systematic reviews of interventions (2nd ed.). John Wiley & Sons.
Hudak, J., Bernat, E. M., Fix, S. T., Prince, K. C., Froeliger, B., & Garland, E. L. (2022). Neurophysiological deficits during reappraisal of negative emotional stimuli in opioid misuse. Biological Psychiatry.
*Hudak, J., Hanley, A. W., Marchand, W. R., Nakamura, Y., Yabko, B., & Garland, E. L. (2021). Endogenous theta stimulation during meditation predicts reduced opioid dosing following treatment with Mindfulness-Oriented Recovery Enhancement. Neuropsychopharmacology, 46(4). https://doi.org/10.1038/s41386-020-00831-4
Ignaszewski, M. J. (2021). The epidemiology of drug abuse. The Journal of Clinical Pharmacology, 61(S2), S10–S17. https://doi.org/10.1002/jcph.1937
Ilgen, M. A., Perron, B., Czyz, E. K., McCammon, R. J., & Trafton, J. (2010). The timing of onset of pain and substance use disorders. The American Journal on Addictions, 19(5), 409–415. https://doi.org/10.1111/j.1521-0391.2010.00065.x
Jakubczyk, A., Ilgen, M. A., Kopera, M., Krasowska, A., Klimkiewicz, A., Bohnert, A., Blow, F. C., Brower, K. J., & Wojnar, M. (2016). Reductions in physical pain predict lower risk of relapse following alcohol treatment. Drug and Alcohol Dependence, 158, 167–171. https://doi.org/10.1016/j.drugalcdep.2015.11.020
Khantzian, E. J. (1997). The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry, 4(5), 231–244. https://doi.org/10.3109/10673229709030550
Kober, H., Mende-Siedlecki, P., Kross, E. F., Weber, J., Mischel, W., Hart, C. L., & Ochsner, K. N. (2010). Prefrontal–striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences, 107(33), 14811–14816.
Koob, G. F. (2021). Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacological Reviews, 73(1), 163–201. https://doi.org/10.1124/pharmrev.120.000083
Kuerbis, A., & Sacco, P. (2013). A review of existing treatments for substance abuse among the elderly and recommendations for future directions. Substance Abuse: Research and Treatment, 7, SART.S7865. https://doi.org/10.4137/SART.S7865
Lappan, S. N., Brown, A. W., & Hendricks, P. S. (2020). Dropout rates of in-person psychosocial substance use disorder treatments: A systematic review and meta-analysis. Addiction, 115(2), 201–217.
Larson, M. J., Paasche-Orlow, M., Cheng, D. M., Lloyd-Travaglini, C., Saitz, R., & Samet, J. H. (2007). Persistent pain is associated with substance use after detoxification: A prospective cohort analysis. Addiction, 102(5), 752–760. https://doi.org/10.1111/j.1360-0443.2007.01759.x
*Li, W., Garland, E. L., McGovern, P., O’Brien, J. E., Tronnier, C., & Howard, M. O. (2017). Mindfulness-oriented recovery enhancement for internet gaming disorder in U adults: A stage I randomized controlled trial. Psychology of Addictive Behaviors Journal of the Society of Psychologists in Addictive Behaviors, 31(4), 393–402. https://doi.org/10.1037/adb0000269
Lipsey, M. W. (2003). Those confounded moderators in meta-analysis: Good, bad, and ugly. The ANNALS of the American Academy of Political and Social Science, 587(1), 69–81. https://doi.org/10.1177/0002716202250791
Manchikanti, L., Giordano, J., Boswell, M. V., Fellows, B., Manchukonda, R., & Pampati, V. (2007). Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. Journal of Opioid Management, 3(2), 89. https://doi.org/10.5055/jom.2007.0045
Moher, D., Liberati, A., & Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement., 6(7), e1000097.
Morasco, B. J., Gritzner, S., Lewis, L., Oldham, R., Turk, D. C., & Dobscha, S. K. (2011). Systematic review of prevalence, correlates, and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorder. PAIN®, 152(3), 488–497. https://doi.org/10.1016/j.pain.2010.10.009
Morris, S. B. (2008). Estimating effect sizes from pretest-posttest-control group designs. Organizational Research Methods, 11(2), 364–386. https://doi.org/10.1177/1094428106291059
Murray, C. J. L., Aravkin, A. Y., Zheng, P., Abbafati, C., Abbas, K. M., Abbasi-Kangevari, M., Abd-Allah, F., Abdelalim, A., Abdollahi, M., Abdollahpour, I., Abegaz, K. H., Abolhassani, H., Aboyans, V., Abreu, L. G., Abrigo, M. R. M., Abualhasan, A., Abu-Raddad, L. J., Abushouk, A. I., Adabi, M., … Lim, S. S. (2020). Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet, 396(10258), 1223–1249https://doi.org/10.1016/S0140-6736(20)30752-2
*Parisi, A., Hanley, A. W., & Garland, E. L. (2022a). Mindfulness-Oriented Recovery Enhancement reduces opioid craving, pain, and negative affect among chronic pain patients on long-term opioid therapy: An analysis of within- and between-person state effects. Behaviour Research and Therapy, 152. Scopus. https://doi.org/10.1016/j.brat.2022a.104066
Parisi, A., Landicho, H. L., Hudak, J., Leknes, S., Froeliger, B., & Garland, E. L. (2022b). Emotional distress and pain catastrophizing predict cue-elicited opioid craving among chronic pain patients on long-term opioid therapy. Drug and Alcohol Dependence, 233, 109361.
Pastor, D. A., & Lazowski, R. A. (2018). On the multilevel nature of meta-analysis: A tutorial, comparison of software programs, and discussion of analytic choices. Multivariate Behavioral Research, 53(1), 74–89. https://doi.org/10.1080/00273171.2017.1365684
Ritchie, H., & Roser, M. (2019). Drug Use. Our World in Data. https://ourworldindata.org/drug-use
*Roberts, R. L., Ledermann, K., & Garland, E. L. (2022). Mindfulness-oriented recovery enhancement improves negative emotion regulation among opioid-treated chronic pain patients by increasing interoceptive awareness. Journal of Psychosomatic Research, 152. Scopus. https://doi.org/10.1016/j.jpsychores.2021.110677
Rogers, A. H., Zvolensky, M. J., Ditre, J. W., Buckner, J. D., & Asmundson, G. J. G. (2021). Association of opioid misuse with anxiety and depression: A systematic review of the literature. Clinical Psychology Review, 84, 101978. https://doi.org/10.1016/j.cpr.2021.101978
Rosenthal, R. (1984). Applied social research methods series, Vol. 6. Meta-analytic procedures for social research.
RStudio Team (2020). RStudio: Integrated development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/.
Sheu, R., Lussier, D., Rosenblum, A., Fong, C., Portenoy, J., Joseph, H., & Portenoy, R. K. (2008). Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Medicine, 9(7), 911–917. https://doi.org/10.1111/j.1526-4637.2008.00420.x
Shurman, J., Koob, G. F., & Gutstein, H. B. (2010). Opioids, pain, the brain, and hyperkatifeia: A framework for the rational use of opioids for pain. Pain Medicine (Malden, Mass.), 11(7), 1092–1098. https://doi.org/10.1111/j.1526-4637.2010.00881.x
Spears, C. A. (2019). Mindfulness-based interventions for addictions among diverse and underserved populations. Current Opinion in Psychology, 30, 11–16. https://doi.org/10.1016/j.copsyc.2018.12.012
Sterne, J. A., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., ... & Higgins, J. P. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ, 366.
Tiffany, S. T. (1990). A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review, 97, 147–168.
Trøstheim, M., Eikemo, M., Meir, R., Hansen, I., Paul, E., Kroll, S. L., Garland, E. L., & Leknes, S. (2020). Assessment of anhedonia in adults with and without mental illness: A systematic review and meta-analysis. JAMA Network Open, 3(8), e2013233–e2013233.
Turner, S., Mota, N., Bolton, J., & Sareen, J. (2018). Self-medication with alcohol or drugs for mood and anxiety disorders: A narrative review of the epidemiological literature. Depression and Anxiety, 35(9), 851–860. https://doi.org/10.1002/da.22771
United Nations Office of Drugs and Crime. (2021). 2021 World drug report. Retrieved from https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html
Van den Noortgate, W., López-López, J. A., Marín-Martínez, F., & Sánchez-Meca, J. (2015). Meta-analysis of multiple outcomes: A multilevel approach. Behavior Research Methods, 47(4), 1274–1294. https://doi.org/10.3758/s13428-014-0527-2
Van Den Noortgate, W., & Onghena, P. (2003). Hierarchical linear models for the quantitative integration of effect sizes in single-case research. Behavior Research Methods, Instruments, & Computers, 35(1), 1–10. https://doi.org/10.3758/BF03195492
van Rijswijk, S. M., van Beek, M. H. C. T., Schoof, G. M., Schene, A. H., Steegers, M., & Schellekens, A. F. (2019). Iatrogenic opioid use disorder, chronic pain and psychiatric comorbidity: A systematic review. General Hospital Psychiatry, 59, 37–50. https://doi.org/10.1016/j.genhosppsych.2019.04.008
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48.
Vowles, K. E., McEntee, M. L., Julnes, P. S., Frohe, T., Ney, J. P., & van der Goes, D. N. (2015). Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain, 156(4), 569–576.
Wilson, N. (2020). Drug and opioid-involved overdose deaths—United States, 2017–2018. MMWR. Morbidity and Mortality Weekly Report, 69.
Woolf, S. H., & Schoomaker, H. (2019). Life expectancy and mortality rates in the United States, 1959–2017. JAMA, 322(20), 1996. https://doi.org/10.1001/jama.2019.16932
Funding
This research was supported by R01DA042033, R21AT010109, and R03DA032517 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
A.P. participated in the design of the meta-analysis, wrote the search protocol and inclusion criteria, conducted literature searches, reviewed all selected studies, extracted study data, conducted meta-analyses, and wrote the first draft of the manuscript. R.L.R participated in the design of the meta-analysis, conducted literature searches, reviewed all selected studies, extracted study data, reviewed results of meta-analyses, and contributed to the final manuscript. A.W.H. and E.L.G. participated in the design of the meta-analysis and contributed to the final manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
Eric Garland, PhD, LCSW, is the Director of the Center on Mindfulness and Integrative Health Intervention Development. The Center provides Mindfulness-Oriented Recovery Enhancement (MORE), mindfulness-based therapy, and cognitive behavioral therapy in the context of research trials for no cost to research participants; however, Dr. Garland has received honoraria and payment for delivering seminars, lectures, and teaching engagements (related to training clinicians in MORE and mindfulness) sponsored by institutions of higher education, government agencies, academic teaching hospitals, and medical centers. Dr. Garland also receives royalties from the sale of books related to MORE. Dr. Garland is also a consultant and licensor to BehaVR, LLC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parisi, A., Roberts, R.L., Hanley, A.W. et al. Mindfulness-Oriented Recovery Enhancement for Addictive Behavior, Psychiatric Distress, and Chronic Pain: A Multilevel Meta-Analysis of Randomized Controlled Trials. Mindfulness 13, 2396–2412 (2022). https://doi.org/10.1007/s12671-022-01964-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12671-022-01964-x