Abstract
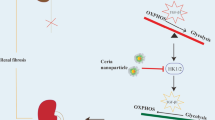
The current study assessed the impact of cerium oxide nanoparticles (nanocerium, NC) on adriamycin (ADR)-induced nephropathy in addition to the physiological role of oxidative stress, Nrf2/HO-1 pathway, apoptosis, TGF-β, and Sirt-1 in its action. Forty adult male Sprague–Dawley rats were divided into four groups as follows: control, NC, ADR, and ADR + NC groups. At the end of the experiment, urine, blood samples, and kidney were taken for assessment of serum creatinine (Cr), blood urea nitrogen (BUN), and urinary protein. Also, malondialdehyde (MDA), reduced glutathione (GSH) and catalase (CAT) levels, histopathological examinations, immunohistochemical examinations for caspase-3, TGF-β, Sirt-1, and real-time PCR for the expression of Nrf2 and HO-1 antioxidant genes in kidney tissues were done. NC significantly improved the raised serum creatinine, BUN, and urinary proteins. Also, NC improved the expression of caspase-3 and markers of oxidative stress in kidney tissues, and it also reduced morphological renal damage. Moreover, NC caused a significant increase in the expression of SIRT1 and Nrf2/HO-1 in kidney tissues. Also, markedly decreased renal fibrosis. Nanocerium alleviates ADR nephropathy, which may be related to the antioxidant and anti-fibrotic action of NC. Also, upregulating and activating SIRT1, Nrf2/HO-1 signaling and reduction of apoptosis.
Graphical Abstract








Similar content being viewed by others
References
Hill, N. R., Fatoba, S. T., Oke, J. L., Hirst, J. A., O’Callaghan, C. A., Lasserson, D. S., & Hobbs, F. R. (2016). Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PloS One, 11(7), e0158765.
Foreman, K. J., Marquez, N., Dolgert, A., Fukutaki, K., Fullman, N., McGaughey, M., & Yuan, C. W. (2018). Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. The Lancet, 392(10159), 2052–2090.
Gansevoort, R. T., Correa-Rotter, R., Hemmelgarn, B. R., Jafar, T. H., Heerspink, H. J. L., Mann, J. F., & Wen, C. P. (2013). Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. The Lancet, 382(9889), 339–352.
Trautmann, A., Schnaidt, S., Lipska-Ziętkiewicz, B. S., Bodria, M., Ozaltin, F., Emma, F., & Oh, J. (2017). Long-term outcome of steroid-resistant nephrotic syndrome in children. Journal of the American Society of Nephrology, 28(10), 3055–3065.
Furuichi, K., Shintani, H., Sakai, Y., Ochiya, T., Matsushima, K., Kaneko, S., & Wada, T. (2012). Effects of adipose-derived mesenchymal cells on ischemia–reperfusion injury in kidney. Clinical and Experimental Nephrology, 16(5), 679–689.
Lee, V. W., & Harris, D. C. (2011). Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology, 16, 30–38.
Sarkaki, A., Badavi, M., Nejaddehbashi, F., Hajipour, S., Basir, Z., & Amini, N. (2023). The renoprotective effects of hesperidin on kidney injury induced by exposure to severe chronic dust storm particulate matter through inhibiting the Smads/TGF-β1 signaling in rat. Naunyn Schmiedebergs Arch Pharmacol, 396(12), 3615–3626. https://doi.org/10.1007/s00210-023-02562-x
Amini, N., Maleki, M., & Badavi, M. (2022). Nephroprotective activity of naringin against chemical-induced toxicity and renal ischemia/reperfusion injury: A review. Avicenna J Phytomed, 12(4), 357–370.
Min, H. S., Cha, J. J., Kim, K., Kim, J. E., Ghee, J. Y., Kim, H., Lee, J. E., Han, J. Y., Jeong, L. S., Cha, D. R., & Kang, Y. S. (2016). Renoprotective effects of a highly selective A3 adenosine receptor antagonist in a mouse model of adriamycin-induced nephropathy. Journal of Korean Medical Science, 31(9), 1403.
Cha, J., Joo, H., Park, J. A., Yoo, J., Ghee, D. R., & Cha (2024). Dapagliflozin does not protect against Adriamycin-Induced kidney Injury in mice. Kidney and Blood Pressure Research, 49(1), 81–90.
El-Sayed, E. S. M., Mansour, A. M., & El‐Sawy, W. S. (2017). Alpha lipoic acid prevents doxorubicin‐induced nephrotoxicity by mitigation of oxidative stress, inflammation, and apoptosis in rats. Journal of Biochemical and Molecular Toxicology, 31(9), e21940.
Afsar, T., Razak, S., Almajwal, A., & Al-Disi, D. (2020). Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; improvement by Acacia Hydaspica tannin-rich ethyl acetate fraction. Saudi Journal of Biological Sciences, 27(9), 2251–2260.
Asaad, G. F., Hassan, A., & Mostafa, R. E. (2021). Anti-oxidant impact of lisinopril and enalapril against acute kidney injury induced by doxorubicin in male Wistar rats: Involvement of kidney injury molecule-1. Heliyon, 7(1), e05985.
Edrees, N. E., Galal, A. A., Monaem, A. R. A., Beheiry, R. R., & Metwally, M. M. (2018). Curcumin alleviates colistin-induced nephrotoxicity and neurotoxicity in rats via attenuation of oxidative stress, inflammation and apoptosis. Chemico-biological Interactions, 294, 56–64.
Songbo, M., Lang, H., Xinyong, C., Bin, X., Ping, Z., & Liang, S. (2019). Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicology Letters, 307, 41–48.
Kim, D. R., Lee, S. Y., Kim, J. S., Kim, Y. G., Moon, J. Y., Lee, S. H., & Jeong, K. H. (2017). Ameliorating effect of gemigliptin on renal injury in murine adriamycin-induced nephropathy. BioMed Research International, 2017. https://doi.org/10.1155/2017/7275109
Soltani Hekmat, A., Chenari, A., Alipanah, H., & Javanmardi, K. (2021). Protective effect of alamandine on doxorubicininduced nephrotoxicity in rats. BMC Pharmacology and Toxicology, 22(1), 1–11.
Wang, Y., Chao, X., Shi, H., Mehboob, H., & Hassan, W. (2019). Phoenix dactylifera protects against doxorubicin-induced cardiotoxicity and nephrotoxicity. Cardiology Research and Practice, 2019.
Celardo, I., Traversa, E., & Ghibelli, L. (2011). Cerium oxide nanoparticles: A promise for applications in therapy. Journal of Experimental Therapeutics & Oncology, 9(1), 47–51.
Pirmohamed, T., Dowding, J. M., Singh, S., Wasserman, B., Heckert, E., Karakoti, A. S., & Self, W. T. (2010). Nanoceria exhibit redox state-dependent catalase mimetic activity. Chemical Communications, 46(16), 2736–2738.
Korsvik, C., Patil, S., Seal, S., & Self, W. T. (2007). Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chemical Communications, 43(10), 1056–1058.
Dowding, J. M., Dosani, T., Kumar, A., Seal, S., & Self, W. T. (2012). Cerium oxide nanoparticles scavenge nitric oxide radical (˙ NO). Chemical Communications, 48(40), 4896–4898.
Asati, A., Santra, S., Kaittanis, C., & Perez, J. M. (2010). Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS nano, 4(9), 5321–5331.
Hussein, A. M., Eldosoky, M., Handhle, A., Elserougy, H., Sarhan, M., Sobh, M. A., & El Nashar, E. M. (2016). Effects of long-acting erythropoietin analog darbepoetin-α on adriamycin-induced chronic nephropathy. International Urology and Nephrology, 48(2), 287–297.
Sangomla, S., Saifi, M. A., Khurana, A., & Godugu, C. (2018). Nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. Journal of Trace Elements in Medicine and Biology, 47, 53–62.
Parasuraman, S., Raveendran, R., & Kesavan, R. (2010). Blood sample collection in small laboratory animals. Journal of Pharmacology & Pharmacotherapeutics, 1(2), 87.
Cruz, J., Loste, J., & Burzaco, O. (1998). Observations on the use of medetomidine/ketamine and its reversal with atipamezole for chemical restraint in the mouse. Laboratory Animals, 32(1), 18–22.
Mohamed Ibrahim, H. A., Mohamed Hussein, A., Gabr, M., El-Saeed, R. A., Abd-Alhakem Ammar, O., Hassan Mosa, A. A., & Abdel-Aziz, A. A. F. (2023). Effect of melatonin on alpha synuclein and autophagy in dopaminergic neuronal differentiation of adipose mesenchymal stem cells. Reports of Biochemistry and Molecular Biology, 13–26.
Savani, M., Woerner, K., Bu, L., Birkenbach, M., & Skubitz, K. M. (2021). Pegylated liposomal doxorubicin-induced renal toxicity in retroperitoneal liposarcoma: A case report and literature review. Cancer Chemotherapy and Pharmacology, 87(2), 289–294.
Bohnert, B. N., & Artunc, F. (2018). Induction of nephrotic syndrome in mice by retrobulbar injection of doxorubicin and prevention of volume retention by sustained release aprotinin. JoVE (Journal of Visualized Experiments)(135), e57642.
Al-Qahtani, W. H., Alshammari, G. M., Alshuniaber, M. A., Husain, M., Alawwad, S. A., Al-Ayesh, S. T., & Aldawood, A. S. (2022). The protective effect of isoliquiritigenin against doxorubicin-induced nephropathy in rats entails activation of Nrf2 signaling as one key mechanism. Journal of King Saud University-Science, 34(6), 102165.
Alagal, R. I., AlFaris, N. A., Alshammari, G. M., ALTamimi, J. Z., AlMousa, L. A., & Yahya, M. A. (2022). Kaempferol attenuates doxorubicin-mediated nephropathy in rats by activating SIRT1 signaling. Journal of Functional Foods, 89, 104918.
Rajasekaran, M. (2019). Nephroprotective effect of Costus pictus extract against doxorubicin-induced toxicity on Wistar rat. Bangladesh Journal of Pharmacology, 14(2), 93–100.
Liu, H. F., Guo, L. Q., Huang, Y. Y., Chen, K., Tao, J. L., Li, S. M., & Chen, X. W. (2010). Thiazolidinedione attenuate proteinuria and glomerulosclerosis in adriamycin-induced nephropathy rats via slit diaphragm protection. Nephrology, 15(1), 75–83.
Montoya, J., Luna, H., Morelos, A., Catedral, M., Lava, A., Amparo, J., & Cristal-Luna, G. (2013). Association of creatinine clearance with neutropenia in breast cancer patients undergoing chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide (FAC). Medical Journal of Malaysia, 68(2), 153–156.
Saifi, M. A., Sangomla, S., Khurana, A., & Godugu, C. (2019). Protective effect of nanoceria on cisplatin-induced nephrotoxicity by amelioration of oxidative stress and pro-inflammatory mechanisms. Biological Trace Element Research, 189(1), 145–156.
Shokrzadeh, M., Jahani, M., Vafaeipour, Z., & Shaki, F. (2016). Protective effect of Nanoceria against renal mitochondrial damage in streptozocine-induced diabetic mice. Journal of Mazandaran University of Medical Sciences, 25(132), 258–269.
Ali, A. A., Saad, E. B., Hassan, R., El-Raouf, O. M., & Gad, A. M. (2022). Mechanistic study on the effect of peroxisome proliferator. Activated receptor agonist drugs against doxorubicin induced nephrotoxicity in rats.Preprint. https://doi.org/10.21203/rs.3.rs-1590975/v1.ResearchSquare
Zhang, Y., Xu, Y., Qi, Y., Xu, L., Yin, L., Tao, X., & Ma, X. (2019). Corrigendum to protective effects of dioscin agains doxorubicin-induced nephrotoxicity via adjusting FXR-mediated oxidative stress and inflammation [Toxicology 378 (2017) 53–64]. Toxicology, 424, 152251.
Kumari, P., Saifi, M. A., Khurana, A., & Godugu, C. (2018). Cardioprotective effects of nanoceria in a murine model of cardiac remodeling. Journal of Trace Elements in Medicine and Biology, 50, 198–208.
Kyosseva, S. V., Chen, L., Seal, S., & McGinnis, J. F. (2013). Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice. Experimental eye Research, 116, 63–74.
González-Flores, D., De Nicola, M., Bruni, E., Caputo, F., Rodriguez, A. B., Pariente, J. A., & Ghibelli, L. (2014). Nanoceria protects from alterations in oxidative metabolism and calcium overloads induced by TNFα and cycloheximide in U937 cells: Pharmacological potential of nanoparticles. Molecular and Cellular Biochemistry, 397(1), 245–253.
Cimini, A., D’Angelo, B., Das, S., Gentile, R., Benedetti, E., Singh, V., & Seal, S. (2012). Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of Aβ aggregates modulate neuronal survival pathways. Acta Biomaterialia, 8(6), 2056–2067.
Khurana, A., Saifi, M., & Godugu, C. (2017). Nanoceria reduces oxidative stress, inflammation and display anti-fibrotic properties in animal models of chronic pancreatitis Paper presented at the PANCREAS.
Abdelhameed, M. F., Bashandy, S. A., Ibrahim, F. A., Morsy, F., Farid, O., & Elbaset, M. A. (2022). The pivotal role of Cerium oxide nanoparticles in thioacetamide-induced hepatorenal injury in rat. Egyptian Journal of Chemistry.
El-Hawary, S. S., Hammam, W. E., El-Tantawi, M. E. M., Yassin, N. A., Kirollos, F. N., Abdelhameed, M. F., & Sobeh, M. (2021). Apple leaves and their major secondary metabolite phlorizin exhibit distinct neuroprotective activities: Evidence from in vivo and in silico studies. Arabian Journal of Chemistry, 14(6), 103188.
Ren, X., Bo, Y., Fan, J., Chen, M., Xu, D., Dong, Y., & Jin, Y. (2016). Dalbergioidin ameliorates doxorubicin-induced renal fibrosis by suppressing the TGF-β signal pathway. Mediators of Inflammation, 2016.
Modelli de Andrade, L. G., Viero, R. M., & de Cordeiro, M. F. (2012). Treatment of adriamycin-induced nephropathy with erythropoietin and G-CSF. Journal of Nephrology, 26(3), 534–539.
Sadek, E. M., Salama, N. M., Ismail, D. I., & Elshafei, A. A. (2016). Histological study on the protective effect of endogenous stem-cell mobilization in adriamycin-induced chronic nephropathy in rats. Journal of Microscopy and Ultrastructure, 4(3), 133–142.
Saifi, M. A., Peddakkulappagari, C. S., Ahmad, A., & Godugu, C. (2020). Leveraging the pathophysiological alterations of obstructive nephropathy to treat renal fibrosis by cerium oxide nanoparticles. ACS Biomaterials Science & Engineering, 6(6), 3563–3573.
Biondo, L. A., Lima Junior, E. A., Souza, C. O., Cruz, M. M., Cunha, R. D., Alonso-Vale, M. I., & Santos, D., R. V (2016). Impact of doxorubicin treatment on the physiological functions of white adipose tissue. PloS One, 11(3), e0151548.
Solgi, T., Amiri, I., Asl, S. S., Saidijam, M., Seresht, B. M., & Artimani, T. (2021). Antiapoptotic and antioxidative effects of cerium oxide nanoparticles on the testicular tissues of streptozotocin-induced diabetic rats: An experimental study. International Journal of Reproductive BioMedicine, 19(7), 589.
Hamzeh, M., Amiri, F. T., Beklar, S. Y., & Hosseinimehr, S. J. (2018). Nephroprotective effect of cerium oxide nanoparticles on cyclophosphamide-induced nephrotoxicity via anti-apoptotic and antioxidant properties in BALB/c mice. Marmara Pharmaceutical Journal, 22(2), 180–189.
Elsherbiny, N. M., & El-Sherbiny, M. (2014). Thymoquinone attenuates Doxorubicin-induced nephrotoxicity in rats: Role of Nrf2 and NOX4. Chemico-biological Interactions, 223, 102–108.
Lin, E. Y., Bayarsengee, U., Wang, C. C., Chiang, Y. H., & Cheng, C. W. (2018). The natural compound 2, 3, 5, 4′-tetrahydroxystilbene‐2‐O‐β‐d glucoside protects against adriamycin‐induced nephropathy through activating the Nrf2‐Keap1 antioxidant pathway. Environmental Toxicology, 33(1), 72–82.
El-Emam, S. Z., Soubh, A. A., Al-Mokaddem, A. K., & Abo El-Ella, D. M. (2020). Geraniol activates Nrf-2/HO-1 signaling pathway mediating protection against oxidative stress-induced apoptosis in hepatic ischemia-reperfusion injury. Naunyn-Schmiedeberg’s Archives of Pharmacology, 393(10), 1849–1858.
Hennig, P., Garstkiewicz, M., Grossi, S., Di Filippo, M., French, L. E., & Beer, H. D. (2018). The crosstalk between Nrf2 and inflammasomes. International Journal of Molecular Sciences, 19(2), 562.
Vomund, S., Schäfer, A., Parnham, M. J., Brüne, B., & Von Knethen, A. (2017). Nrf2, the master regulator of anti-oxidative responses. International Journal of Molecular Sciences, 18(12), 2772.
Hasanvand, D., Amiri, I., Soleimani Asl, S., Saidijam, M., Shabab, N., & Artimani, T. (2018). Effects of CeO2 nanoparticles on the HO-1, NQO1, and GCLC expression in the testes of diabetic rats. Canadian Journal of Physiology and Pharmacology, 96(9), 963–969.
Taha, M., Elazab, S. T., Badawy, A. M., Saati, A. A., Qusty, N. F., Al-Kushi, A. G., & Farage, A. E. (2022). Activation of SIRT-1 pathway by Nanoceria sheds light on its ameliorative effect on doxorubicin-induced cognitive impairment (Chemobrain): Restraining its neuroinflammation, synaptic dysplasticity and apoptosis. Pharmaceuticals, 15(8), 918.
Song, S., Chu, L., Liang, H., Chen, J., Liang, J., Huang, Z., & Chen, X. (2019). Protective effects of dioscin against doxorubicin-induced hepatotoxicity via regulation of Sirt1/FOXO1/NF-κb signal. Frontiers in Pharmacology, 10, 1030.
Zhang, J., Bi, R., Meng, Q., Wang, C., Huo, X., Liu, Z., & Ma, X. (2019). Catalpol alleviates adriamycin-induced nephropathy by activating the SIRT1 signalling pathway in vivo and in vitro. British Journal of Pharmacology, 176(23), 4558–4573.
Shati, A. A., & El-Kott, A. F. (2021). Acylated ghrelin protects against doxorubicin‐induced nephropathy by activating silent information regulator 1. Basic & Clinical Pharmacology & Toxicology, 128(6), 805–821.
Funding
None.
Author information
Authors and Affiliations
Contributions
F.H.E, A. A.K., E.A.E., M.T., M. E., A. A., and A.M.H. wrote the main manuscript text, E.A.E. and M.T. did statistical analyses, and F.H.E., A.A., and A.M.H. prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The experimental protocol of this experimental study was approved by Mansoura University-Animal Care and Use Committee with the code # MU-ACUC, MED.R.23.08.21). Informed consent is not applicable.
Informed Consent
None.
Competing Interests
The authors declare no competing interests.
Research Involving Humans and Animals Statement
This work was animal research not a human research that was approved by our university ethical comitte for animal care and use with code #MU-ACUC, MED.R.23.08.21.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elsaid, F.H., Khalil, A.A., Eid, E.A. et al. Effect of Cerium Oxide Nanoparticles on Adriamycin-Induced Nephropathy: Possible Role for Nrf2/HO-1 and TGF-β/Sirt-1 Pathways. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01448-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01448-3