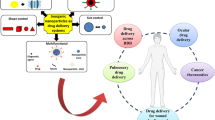
Abstract
Due to low toxicity and anti-cancer activity, selenium nanoparticles have been studied to treat various cancers. In the present study, curcumin-loaded selenium-chitosan nanocomposites (CUR-Cs-SeNCs) were prepared, and their inhibitory effect on 4T1 cell line and tumor-bearing mice was reported. FTIR, XRD, EDX, AFM, and SEM analysis confirmed the successful preparation of CUR-Cs-SeNCs. The average size, polydispersity index, and zeta potential of CUR-Cs-SeNCs were 167.43 ± 6.52, 0.18 ± 0.05, and 35.62 ± 5.21 mV, respectively. The cumulative released percentage of curcumin from nanocomposites at pH 5.5 was about 1.8 times higher than at pH 7.4. Moreover, CUR-Cs-SeNCs showed a diffusion-controlled release pattern at pH 6.8 and 5.5. The in vitro cytotoxicity study on 4T1 cells revealed that CUR-Cs-SeNCs dramatically reduced cell proliferation rate compared to pure curcumin. Additionally, the in vivo study confirmed that the CUR-Cs-SeNCs were more successful in reducing the tumor volume than net CUR. More importantly, histopathological studies revealed a more substantial inhibitory effect on tumor growth and liver metastasis of CUR-Cs-SeNCs as compared to free curcumin. No significant signs of toxicity were detected in the vital organs of CUR-Cs-SeNCs-receiving animals. Therefore, considering the pH-dependent release, the improved inhibitory effect on turmeric cells, and the outstanding performance in the animal model, the CUR-Cs-SeNCs may be promising in future tumor management.







Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Miller, K. D., et al. (2021). Cancer statistics for the US Hispanic/Latino population. CA: A Cancer Journal for Clinicians, 71(6), 466–487.
Carty, N., Foggitt, A., Hamilton, C., Royle, G., & Taylor, I. (1995). Patterns of clinical metastasis in breast cancer: An analysis of 100 patients. European Journal of Surgical Oncology (EJSO), 21(6), 607–608.
Kumar, A., et al. (2020). Deep feature learning for histopathological image classification of canine mammary tumors and human breast cancer. Information Sciences, 508, 405–421.
El-Hussein, A., Manoto, S. L., Ombinda-Lemboumba, S., Alrowaili, Z. A., & Mthunzi-Kufa, P. (2021). A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 21(2), 149–161.
Zoi, V., Galani, V., Lianos, G. D., Voulgaris, S., Kyritsis, A. P., & Alexiou, G. A. (2021). The role of curcumin in cancer treatment. Biomedicines, 9(9), 1086.
Goycoolea, F. M., & Milkova, V. (2017). Electrokinetic behavoir of chitosan adsorbed on o/w nanoemulsion droplets. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 519, 205–211.
Helzlsouer, K. J., et al. (2000). Association between α-tocopherol, γ-tocopherol, selenium, and subsequent prostate cancer. JNCI: Journal of the National Cancer Institute, 92(24), 2018–2023.
Kong, L., et al. (2011). The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials, 32(27), 6515–6522.
Liu, T., et al. (2020). Selenium nanoparticles regulates selenoprotein to boost cytokine-induced killer cells-based cancer immunotherapy. Nano Today, 35, 100975.
Micke, O., Schomburg, L., Buentzel, J., Kisters, K., & Muecke, R. (2009). Selenium in oncology: From chemistry to clinics. Molecules, 14(10), 3975–3988.
Liu, T., Zeng, L., Jiang, W., Fu, Y., Zheng, W., & Chen, T. (2015). “Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells,” Nanomedicine: Nanotechnology. Biology and Medicine, 11(4), 947–958.
Varlamova, E. G., et al. (2021). Mechanisms of the cytotoxic effect of selenium nanoparticles in different human cancer cell lines. International Journal of Molecular Sciences, 22(15), 7798.
Ren, Y., et al. (2013). Antitumor activity of hyaluronic acid–selenium nanoparticles in Heps tumor mice models. International Journal of Biological Macromolecules, 57, 57–62.
Sonkusre, P., & Cameotra, S. S. (2017). Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. Journal of Nanobiotechnology, 15(1), 1–12.
Islam, W., Niidome, T., & Sawa, T. (2022). Enhanced permeability and retention effect as a ubiquitous and epoch-making phenomenon for the selective drug targeting of solid tumors. Journal of Personalized Medicine, 12(12), 1964.
Wang, Y., et al. (2015). Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food and Chemical Toxicology, 85, 71–77.
Wang, X., Sun, K., Tan, Y., Wu, S., & Zhang, J. (2014). Efficacy and safety of selenium nanoparticles administered intraperitoneally for the prevention of growth of cancer cells in the peritoneal cavity. Free Radical Biology and Medicine, 72, 1–10.
Li, Y., et al. (2020). A pH-sensitive drug delivery system based on folic acid-targeted HBP-modified mesoporous silica nanoparticles for cancer therapy. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 590, 124470.
de Oliveira Silva, J., et al. (2019). Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomedicine & Pharmacotherapy, 118, 109323.
Anirudhan, T., Mohan, M., & Rajeev, M. (2022). Modified chitosan-hyaluronic acid based hydrogel for the pH-responsive Co-delivery of cisplatin and doxorubicin. International Journal of Biological Macromolecules, 201, 378–388.
Fathi, M., Alami-Milani, M., Geranmayeh, M. H., Barar, J., Erfan-Niya, H., & Omidi, Y. (2019). Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly (N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. International Journal of Biological Macromolecules, 128, 957–964.
Chen, W., Li, Y., Yang, S., Yue, L., Jiang, Q., & Xia, W. (2015). Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydrate Polymers, 132, 574–581.
Yu, S., et al. (2021). Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: An in vitro and in vivo study. Colloids and Surfaces B: Biointerfaces, 203, 111759.
Qin, Y.-T., Peng, H., He, X.-W., Li, W.-Y., & Zhang, Y.-K. (2019). pH-responsive polymer-stabilized ZIF-8 nanocomposites for fluorescence and magnetic resonance dual-modal imaging-guided chemo-/photodynamic combinational cancer therapy. ACS Applied Materials & Interfaces, 11(37), 34268–34281.
Hashemi-Moghaddam, H., Kazemi-Bagsangani, S., Jamili, M., & Zavareh, S. (2016). Evaluation of magnetic nanoparticles coated by 5-fluorouracil imprinted polymer for controlled drug delivery in mouse breast cancer model. International Journal of Pharmaceutics, 497(1–2), 228–238.
Yilmaz, M. T., Ispirli, H., Taylan, O., & Dertli, E. (2021). A green nano-biosynthesis of selenium nanoparticles with Tarragon extract: Structural, thermal, and antimicrobial characterization. Lwt, 141, 110969.
Chen, W., Yue, L., Jiang, Q., Liu, X., & Xia, W. (2018). Synthesis of varisized chitosan-selenium nanocomposites through heating treatment and evaluation of their antioxidant properties. International Journal Biological Macromolecules, 114, 751–758.
Li, Q., Chen, J., Luo, S., Xu, J., Huang, Q., & Liu, T. (2015). Synthesis and assessment of the antioxidant and antitumor properties of asymmetric curcumin analogues. European Journal of Medicinal Chemistry, 93, 461–469.
Gao, X., Zhang, J., & Zhang, L. (2002). Hollow sphere selenium nanoparticles: Their in-vitro anti hydroxyl radical effect. Advanced Materials, 14(4), 290–293.
Dumore, N. S., & Mukhopadhyay, M. (2020). Antioxidant properties of aqueous selenium nanoparticles (ASeNPs) and its catalysts activity for 1, 1-diphenyl-2-picrylhydrazyl (DPPH) reduction. Journal of Molecular Structure, 1205, 127637.
Zhang, Y., Wang, J., & Zhang, L. (2010). Creation of highly stable selenium nanoparticles capped with hyperbranched polysaccharide in water. Langmuir, 26(22), 17617–17623.
Bahrami, B., et al. (2017). Nanoparticles and targeted drug delivery in cancer therapy. Immunology Letters, 190, 64–83. https://doi.org/10.1016/j.imlet.2017.07.015
Zhang, C., Zhai, X., Zhao, G., Ren, F., & Leng, X. (2015). Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydrate Polymers, 134, 158–166.
Song, P., Lu, Z., Jiang, T., Han, W., Chen, X., & Zhao, X. (2022). Chitosan coated pH/redox-responsive hyaluronic acid micelles for enhanced tumor targeted co-delivery of doxorubicin and siPD-L1. International Journal of Biological Macromolecules, 222, 1078–1091. https://doi.org/10.1016/j.ijbiomac.2022.09.245
Adimoolam, M. G., Amreddy, N., Nalam, M. R., & Sunkara, M. V. (2018). A simple approach to design chitosan functionalized Fe3O4 nanoparticles for pH responsive delivery of doxorubicin for cancer therapy. Journal of Magnetism and Magnetic Materials, 448, 199–207. https://doi.org/10.1016/j.jmmm.2017.09.018
Karimifard, S., et al. (2022). pH-Responsive chitosan-adorned niosome nanocarriers for co-delivery of drugs for breast cancer therapy. ACS Applied Nano Materials, 5(7), 8811–8825. https://doi.org/10.1021/acsanm.2c00861
Unsoy, G., Khodadust, R., Yalcin, S., Mutlu, P., & Gunduz, U. (2014). Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. European Journal of Pharmaceutical Sciences, 62, 243–250.
Nogueira, D. R., Tavano, L., Mitjans, M., Pérez, L., Infante, M. R., & Vinardell, M. P. (2013). In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials, 34(11), 2758–2772.
Unsoy, G., Yalcin, S., Khodadust, R., Mutlu, P., Onguru, O., & Gunduz, U. (2014). Chitosan magnetic nanoparticles for pH responsive Bortezomib release in cancer therapy. Biomedicine & Pharmacotherapy, 68(5), 641–648.
Lotfi, S., Bahari, A., & Mahjoub, S. (2019). In vitro biological evaluations of Fe3O4 compared with core–shell structures of chitosan-coated Fe3O4 and polyacrylic acid-coated Fe3O4 nanoparticles. Research on Chemical Intermediates, 45(6), 3497–3512. https://doi.org/10.1007/s11164-019-03804-5
Ferreira, L. M., et al. (2015). Ketoprofen-loaded pomegranate seed oil nanoemulsion stabilized by pullulan: Selective antiglioma formulation for intravenous administration. Colloids and Surfaces B: Biointerfaces, 130, 272–277.
Nurgali, K., Jagoe, R. T., & Abalo, R. (2018). Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Frontiers in Pharmacology, 9, 245.
Chen, Q., et al. (2022). Dual-pH responsive chitosan nanoparticles for improving in vivo drugs delivery and chemoresistance in breast cancer. Carbohydrate Polymers, 290, 119518.
Menon, S., Ks, S. D., Santhiya, R., Rajeshkumar, S., & Kumar, V. (2018). Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids and Surfaces B: Biointerfaces, 170, 280–292.
Shakibaie, M., Shahverdi, A. R., Faramarzi, M. A., Hassanzadeh, G. R., Rahimi, H. R., & Sabzevari, O. (2013). Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharmaceutical Biology, 51(1), 58–63.
Gangadoo, S., Stanley, D., Hughes, R. J., Moore, R. J., & Chapman, J. (2017). The synthesis and characterisation of highly stable and reproducible selenium nanoparticles. Inorganic and Nano-Metal Chemistry, 47(11), 1568–1576.
Lin, Z., et al. (2017). Inhibition of H1N1 influenza virus by selenium nanoparticles loaded with zanamivir through p38 and JNK signaling pathways. RSC Advances, 7(56), 35290–35296.
Acknowledgements
All procedures were conducted according to the Ethical Guidelines for Research, Zanjan University of Medical Sciences University, Zanjan, Iran (Ethical Codes: IR.ZUMS.REC.1399.343 and IR.ZUMS.AEC.1402.029). This research was financially supported by Zanjan University of Medical Sciences, Zanjan, Iran (Grant No: A-12-1381-3) and Trita Nanomedicine Research & Technology Development Center (TNRTC).
Funding
This research was financially supported by Zanjan University of Medical Sciences, Zanjan, Iran (Grant No: A-12–1381-3) and Trita Nanomedicine Research & Technology Development Center (TNRTC).
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution to the paper as follows: study conception and design: Z.K., M.H., S.G.; data collection: E.A., N.S., M.S., Z.K., M.M; analysis and interpretation of results: Z.K., M.H.; draft manuscript preparation: Z.K., F.S.Z., M.K. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
All procedures were conducted according to the Ethical Guidelines for Research, Zanjan University of Medical Sciences University, Zanjan, Iran (Ethical Codes: IR.ZUMS.REC.1399.343 and IR.ZUMS.AEC.1402.029).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karami, Z., Aghajani, E., Salami, M. et al. Enhanced Antitumor and Antimetastatic Activity of pH-Responsive Curcumin-Loaded Chitosan-Selenium Nanocomposites against Breast Cancer. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01431-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01431-y