Abstract
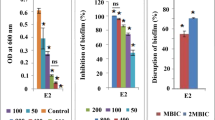
Antimicrobial resistance is a significant concern in aquaculture, prompting the exploration of nanoemulsions (NEs) with a non-resistant mode of action as a safer and more effective alternative to antibiotics. In Asian countries, the culture of Penaeus vannamei has faced growing concerns as V. parahaemolyticus (Vp) has been increasingly linked to shrimp diseases, leading to huge economic losses. In response, stable ozonated oil-in-water NEs (NE-25, 26, 28, 29) were produced using a microfluidization method and characterized by their polydispersity index and average droplet size (NE-25, 26, 28, 29), which were found to 0.108, 0.251, 0.223, 0.173, and 229.7nm, 190.2nm, 201.8nm, and 145.8nm, respectively. The therapeutic potential of NEs is tested against pathogenic strains having tdh and toxR virulence gene, which were isolated from diseased shrimp (VP-S7 and VP-S14), these were found to be more resistant compared to commercial V. parahaemolyticus (MTCC-451) strain. Our NEs confirmatory antibacterial tests include bacteriostatic, bactericidal, antibiofilm, adherence, live/dead assays, and oxygen consumption rate (OCR) studies. All tested nanoemulsions showed effectiveness against planktonic and biofilm stages of V. parahaemolyticus; NE-25 showed strong adherence inhibition, with results of VP-S7 (49.55%), VP-S14 (53.47%), and MTCC-451 (68.76%). The biofilm inhibition of NE-25 (55.97%) is comparable to inhibition with antibiotics gentamicin (58.73%). The NE OCR results (1 × 107 cell/ml) compared to antibiotics treated and untreated, the fold reduction for NE-28 (0.2) is highly significant when compared with commercial antibiotics (0.6) and the growth control (1.0). Therefore, using NEs, which possess high antibacterial properties, is a promising alternative for treating V. parahaemolyticus compared to current antibiotics.









Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Kooloth, V. R., Stentiford, G. D., & Bass, D. (2021). The rise of the syndrome–sub-optimal growth disorders in farmed shrimp. Reviews in Aquaculture, 13, 41888–41906. https://doi.org/10.1111/raq.12550
Little, D. C., Newton, R. W., & Beveridge, M. C. M. (2016). Aquaculture: a rapidly growing and significant source of sustainable food? Status, transitions and potential. Proceedings of the Nutrition Society, 75(3), 274–286. https://doi.org/10.1017/S0029665116000665
Albalat, A., Zacarias, S., Coates, C. J., Neil, D. M., & Planellas, S. R. (2022). Welfare in farmed decapod crustaceans, with particular reference to Penaeus vannamei. Frontiers in Marine Science, 09, 886024. https://doi.org/10.3389/fmars.2022.886024
Ghosh, A. K., Panda, S. K., & Luyten, W. (2021). Anti-vibrio and immune-enhancing activity of medicinal plants in shrimp: A comprehensive review. Fish & Shellfish Immunology, 117, 192–210. https://doi.org/10.1016/j.fsi.2021.08.006
Gunalan, B., Soundarapandian, P., Anand, T., Kotiya, A. S., & Simon, N. T. (2014). Disease occurrence in Litopenaeus vannamei shrimp culture systems in different geographical regions of India. International Journal of Aquatic Biology, 10, 4. https://doi.org/10.5376/ija.2014.04.0004
Tan, L. T. H., Chan, K. G., Lee, L. H., & Goh, B. H. (2016). Streptomyces bacteria as potential probiotics in aquaculture. Frontiers in Microbiology, 7, 79. https://doi.org/10.3389/fmicb.2016.00079
Letchumanan, V., Chan, K. G., & Lee, L. H. (2014). Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Frontiers in Microbiology, 5, 705. https://doi.org/10.3389/fmicb.2014.00705
Wang, L., Chen, Y., Huang, H., Huang, Z., Chen, H., & Shao, Z. (2015). Isolation and identification of Vibrio campbellii as a bacterial pathogen for luminous vibriosis of Litopenaeus vannamei. Aquaculture Research, 46(2), 395–404. https://doi.org/10.1111/are.12191
Raja, R. A., Sridhar, R., Balachandran, C., Palanisammi, A., Ramesh, S., & Nagarajan, K. (2017). Prevalence of Vibrio spp. with special reference to Vibrio parahaemolyticus in farmed penaeid shrimp Penaeus vannamei (Boone, 1931) from selected districts of Tamil Nadu, India. Indian Journal of Fisheries, 64(3):122–128. https://doi.org/10.21077/ijf.2017.64.3.69011-18
Narayanan, S. V., Joseph, T. C., Peeralil, S., Koombankallil, R., Vaiyapuri, M., Mothadaka, M. P., & Lalitha, K. V. (2020). Tropical shrimp aquaculture farms harbour pathogenic Vibrio parahaemolyticus with high genetic diversity and Carbapenam resistance. Marine Pollution Bulletin, 160, 111551. https://doi.org/10.1016/j.marpolbul.2020.111551
Kumar, B. K., Deekshit, V. K., Raj, J. R. M., Rai, P., Shivanagowda, B. M., Karunasagar, I., & Karunasagar, I. (2014). Diversity of Vibrio parahaemolyticus associated with disease outbreak among cultured Litopenaeus vannamei (Pacific white shrimp) in India. Aquaculture, 433, 247–251. https://doi.org/10.1016/j.aquaculture.2014.06.016
Kumar, R., Tung, T. C., Ng, T. H., Chang, C. C., Chen, Y. L., Chen, Y. M., Lin, S. S., & Wang, H. C. (2021). Metabolic alterations in shrimp stomach during acute hepatopancreatic necrosis disease and effects of taurocholate on Vibrio parahaemolyticus. Frontiers in Microbiology, 12, 631468. https://doi.org/10.3389/fmicb.2021.631468
Ahmed, J., Khan, M. H., Unnikrishnan, S., & Ramalingam, K. (2022). Acute hepatopancreases necrosis diseases (AHPND) as challenging threat in shrimp. Biointerface Research in Applied Chemistry, 12(1), 978–991. https://doi.org/10.33263/briac121.978991
Dash, P., Avunje, S., Tandel, R. S., KP, S., & Panigrahi, A. (2017). Biocontrol of luminous vibriosis in shrimp aquaculture: A review of current approaches and future perspectives. Reviews in Fisheries Science & Aquaculture, 25(3), 245–255. https://doi.org/10.1080/23308249.2016.1277973
Okocha, R. C., Olatoye, I. O., & Adedeji, O. B. (2018). Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Reviews, 39(1), 1–22. https://doi.org/10.1186/s40985-018-0099-2
Alloul, A., Wille, M., Lucenti, P., Bossier, P., Van Stappen, G., & Vlaeminck, S. E. (2021). Purple bacteria as added-value protein ingredient in shrimp feed: Penaeus vannamei growth performance, and tolerance against Vibrio and ammonia stress. Aquaculture, 530, 735788. https://doi.org/10.1016/j.aquaculture.2020.735788
Kumar, M., Bishnoi, R. S., Shukla, A. K., & Jain, C. P. (2019). Techniques for formulation of nanoemulsion drug delivery system: A review. Preventive Nutrition and Food Science, 24(3), 225. https://doi.org/10.3746/pnf.2019.24.3.225
Hosseini, S. F., Ramezanzade, L., & McClements, D. J. (2021). Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends in Food Science & Technology, 109, 322–339. https://doi.org/10.1016/j.tifs.2021.01.045
Karthik, P., Ezhilarasi, P. N., & Anandharamakrishna, C. (2017). Challenges associated in stability of food grade nanoemulsions. Critical Reviews in Food Science and Nutrition, 57(7), 1435–1450. https://doi.org/10.1080/10408398.2015.1006767
Madene, A., Jacquot, M., Scher, J., & Desobry, S. (2006). Flavour encapsulation and controlled release–A review. International Journal of Food Science & Technology, 41(1), 1–21. https://doi.org/10.1111/j.1365-2621.2005.00980.x
Zimet, P., Rosenberg, D., & Livney, Y. D. (2011). Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocolloids, 25(5), 1270–1276. https://doi.org/10.1016/j.foodhyd.2010.11.025
Belhaj, N., Arab-Tehrany, E., & Linder, M. (2010). Oxidative kinetics of salmon oil in bulk and in nanoemulsion stabilized by marine lecithin. Process Biochemistry, 45(2), 187–195. https://doi.org/10.1016/j.procbio.2009.09.005
Yuan, Y., Gao, Y., Zhao, J., & Mao, L. (2008). Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Research International, 41(1), 61–68. https://doi.org/10.1016/j.foodres.2007.09.006
Wang, X., Jiang, Y., Wang, Y. W., Huang, M. T., Ho, C. T., & Huang, Q. (2008). Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chemistry, 108(2), 419–424. https://doi.org/10.1016/j.foodchem.2007.10.086
Lawrence, M. J., & Rees, G. D. (2012). Microemulsion-based media as novel drug delivery systems. Advanced Drug Delivery Reviews, 64, 175–193. https://doi.org/10.1016/j.addr.2012.09.018
Teixeira, P. C., Leite, G. M., Domingues, R. J., Silva, J., Gibbs, P. A., & Ferreira, J. P. (2007). Antimicrobial effects of a microemulsion and a nanoemulsion on enteric and other pathogens and biofilms. International Journal of Microbiology, 118(1), 15–19. https://doi.org/10.1016/j.ijfoodmicro.2007.05.008
Ramalingam, K., Amaechi, B. T., Ralph, R. H., & Lee, V. A. (2012). Antimicrobial activity of nanoemulsion on cariogenic planktonic and biofilm organisms. Archives of Oral Biology, 57(1), 15–22. https://doi.org/10.1016/j.archoralbio.2011.07.001
Jangam, A. K., Bhuvaneswari, T., Krishnan, A. N., Katneni, V. K., Avunje, S., Grover, M., Kumar, S., Alavandi, S. V., & Vijayan, K. K. (2018). Draft genome sequence of Vibrio parahaemolyticus strain VP14, isolated from a Penaeus vannamei culture farm. Genome Announcements, 6(11), 00149–00218. https://doi.org/10.1128/genomeA.00149-18
Li, W., Chen, H., He, Z., Han, C., Liu, S., & Li, Y. (2015). Influence of surfactant and oil composition on the stability and antibacterial activity of eugenol nanoemulsions. LWT-Food Science and Technology, 62(1), 39–47. https://doi.org/10.1016/j.lwt.2015.01.012
Kaur, H., Pancham, P., Kaur, R., Agarwal, S., & Singh, M. (2020). Synthesis and characterization of Citrus limonum essential oil based nanoemulsion and its enhanced antioxidant activity with stability for transdermal application. Journal of Biomaterials and Nanobiotechnology, 11(4), 215–236. https://doi.org/10.4236/jbnb.2020.114014
Gholipourkanani, H., Buller, N., & Lymbery, A. (2019). In vitro antibacterial activity of four nano-encapsulated herbal essential oils against three bacterial fish pathogens. Aqua Research, 50(3), 871–875. https://doi.org/10.1111/are.13959
Ramalingam, K., & Lee, V. (2019). Biotic and abiotic substrates for enhancing Acinetobacter baumannii biofilm formation: New approach using extracellular matrix and slanted coverslip technique. The Journal of General and Applied Microbiology, 65(2), 64–71. https://doi.org/10.2323/jgam.2018.05.004
Wang, X. H., & Leung, K. Y. (2000). Biochemical characterization of different types of adherence of Vibrio species to fish epithelial cells. Microbiology, 146(4), 989–998. https://doi.org/10.1099/00221287-146-4-989
Khan, M. H., & Ramalingam, K. (2019). Synthesis of antimicrobial nanoemulsions and its effectuality for the treatment of multi-drug resistant ESKAPE pathogens. Biocatalysis and Agricultural Biotechnology, 18, 101025. https://doi.org/10.1016/j.bcab.2019.101025
Xie, T., Liao, Z., Lei, H., Fang, X., Wang, J., & Zhong, Q. (2017). Antibacterial activity of food-grade chitosan against Vibrio parahaemolyticus biofilms. Microbiology in Clinical Pathology, 110, 291–297. https://doi.org/10.1016/j.micpath.2017.07.011
Stiefel, P., Schmidt-Emrich, S., Maniura-Weber, K., & Ren, Q. (2015). Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiology, 15, 1–9. https://doi.org/10.1186/s12866-015-0376-x
Cal-Sabater, P., Caro, I., Castro, M. J., Cao, M. J., Mateo, J., & Quinto, E. J. (2019). Flow cytometry to assess the counts and physiological state of Cronobacter sakazakii cells after heat exposure. Foods, 8(12), 688. https://doi.org/10.3390/foods8120688
Aashique, M., Roy, A., Kosuru, R. Y., & Bera, S. (2021). Membrane depolarization sensitizes Pseudomonas aeruginosa against tannic acid. Current Microbiology, 78, 713–717. https://doi.org/10.1007/s00284-020-02330-7
Yuan, Y., Gao, Y., Zhao, J., & Mao, L. (2008). Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Research International, 41(1), 61–68. https://doi.org/10.1016/j.foodres.2007.09.006
Rahnama, M., Anvar, S. A., Ahari, H., & Kazempoor, R. (2021). Antibacterial effects of extracted corn zein with garlic extract-based nanoemulsion on the shelf life of Vannamei prawn (Litopenaeus vannamei) at refrigerated temperature. Journal of Food Science, 86(11), 4969–4990. https://doi.org/10.1111/1750-3841.15923
Horison, R., Sulaiman, F. O., Alfredo, D., & Wardana, A. A. (2019). Physical characteristics of nanoemulsion from chitosan/nutmeg seed oil and evaluation of its coating against microbial growth on strawberry. Food Research, 3(6), 821–827. https://doi.org/10.26656/fr.2017.3(6).159
Rohman, A., & Man, Y. C. (2010). Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Research International, 43(3), 886–892. https://doi.org/10.1016/j.foodres.2009.12.006
Osanloo, M., Firooziyan, S., Abdollahi, A., Hatami, S., Nematollahi, A., Elahi, N., & Zarenezhad, E. (2022). Nanoemulsion and nanogel containing Artemisia dracunculus essential oil; larvicidal effect and antibacterial activity. BMC Research Notes, 15(1), 276. https://doi.org/10.1186/s13104-022-06135-8
de Meneses, A. C., Sayer, C., Puton, B. M., Cansian, R. L., Araújo, P. H., & de Oliveira, D. (2019). Production of clove oil nanoemulsion with rapid and enhanced antimicrobial activity against gram-positive and gram-negative bacteria. Journal of Water Process Engineering, 42(6), 13209. https://doi.org/10.1111/jfpe.13209
Pérez-Córdoba, L. J., Norton, I. T., Batchelor, H. K., Gkatzionis, K., Spyropoulos, F., & Sobral, P. J. (2018). Physico-chemical, antimicrobial and antioxidant properties of gelatin-chitosan based films loaded with nanoemulsions encapsulating active compounds. Food Hydrocolloids, 79, 544–559. https://doi.org/10.1016/j.foodhyd.2017.12.012
Hwang, Y. Y., Ramalingam, K., Bienek, D. R., Lee, V., You, T., & Alvarez, R. (2013). Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant Acinetobacter baumannii. Antimicrobial agents and chemotherapy, 57(8), 3568–3575. https://doi.org/10.1128/aac.02109-12
Karan, S., Choudhury, D., & Dixit, A. (2021). Immunogenic characterization and protective efficacy of recombinant CsgA, major subunit of curli fibers, against Vibrio parahaemolyticus. Applied Microbiology and Biotechnology, 105, 599–616. https://doi.org/10.1007/s00253-020-11038-4
Yeh, Y. C., Huang, T. H., Yang, S. C., Chen, C. C., & Fang, J. Y. (2020). Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: A review of recent advances. Frontiers in chemistry, 8, 286. https://doi.org/10.3389/fchem.2020.00286
Roy, P. K., Park, S. H., Song, M. G., & Park, S. Y. (2022). Antimicrobial Efficacy of Quercetin against Vibrio parahaemolyticus biofilm on food surfaces and downregulation of virulence genes. Polymers, 14(18), 3847. https://doi.org/10.3390/polym14183847
Liu, H., Zhu, W., Cao, Y., Gao, J., Jin, T., Qin, N., & Xia, X. (2022). Punicalagin inhibits biofilm formation and virulence gene expression of Vibrio parahaemolyticus. Food Control, 139, 109045. https://doi.org/10.1016/j.foodcont.2022.109045
Sahli, C., Moya, S. E., Lomas, J. S., Gravier-Pelletier, C., Briandet, R., & Hémadi, M. (2022). Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics, 12(5), 2383. https://doi.org/10.7150/thno.67296
Deng, Y., Wang, L., Chen, Y., & Long, Y. (2020). Optimization of staining with SYTO 9/propidium iodide: Interplay, kinetics and impact on Brevibacillus brevis. Biotechniques, 69(2), 88–98. https://doi.org/10.2144/btn-2020-0036
Kosuru, R. Y., Aashique, M., Fathima, A., Roy, A., & Bera, S. (2018). Revealing the dual role of gallic acid in modulating ampicillin sensitivity of Pseudomonas aeruginosa biofilms. Future Microbiology, 13(3), 297–312. https://doi.org/10.2217/fmb-2017-0132
Ahmed, J., Navabshan, I., Unnikrishnan, S., Radhakrishnan, L., Vasagam, K. K., & Ramalingam, K. (2023). In silico and In vitro investigation of phytochemicals against shrimp AHPND syndrome causing PirA/B toxins of vibrio parahaemolyticus. Applied Biochemistry and Biotechnology, 195(12), 7176–7196. https://doi.org/10.1007/s12010-023-04458-1
Tavares, T. M. B., Almeida, H. M. D. E. S., Lage, M. V. M., de Carvalho Feitosa, R., & da Silva Júnior, A. A. (2023). Nanoemulsions: A promising strategy in the fight against bacterial infections. In Medical sciences forum (Vol. 24, No. 1, p. 18). MDPI. https://doi.org/10.3390/ECA2023-16402
Acknowledgements
We acknowledge B. S. Abdur Rahman Crescent Institute of Science and Technology for providing the facility to perform this research; and Dr. Sneha Unnikrishnan for data curation, formal analysis, and investigation. We thank Dr. Soumen Bera and Mr Md. Aashique for their help in doing OCR studies using the Clarke electrode-polygraph instrument.
Funding
This work was supported by the Indian Council of Medical Research [Project ID: 2020–4964].
Author information
Authors and Affiliations
Contributions
Jahangir Ahmed: All experimental work, data curation, formal analysis, investigation, methodology, and writing of the original draft. Dr. Karthikeyan Ramalingam: conceptualization, supervision; validation; writing, review, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Research Involving Humans and Animals Statement
None.
Informed Consent
Not applicable.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors read and agreed to the manuscript for publication.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, J., Ramalingam, K. A Study on the Antipathogenic Effects of Nanoemulsion Against V. parahaemolyticus in Shrimp Aquaculture: Antibacterial and Antibiofilm Activities. BioNanoSci. (2024). https://doi.org/10.1007/s12668-024-01375-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12668-024-01375-3