Abstract
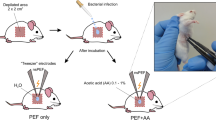
Managing chronic non-healing wounds (CNW) is a time-consuming process accompanied by economic loss. The combination of drug-resistant microflora that is able to organize in a biofilm is the main factor of wound chronicity that requires novel treatment strategies. The current study presents the results of silver nanoparticles (AgNPs) application accompanied by low-frequency ultrasound (US) in the rat model of a purulent CNW. As a result, using AgNPs and low-frequency US demonstrated positive dynamics of wound healing and a reduction in all phases of the wound process. The combined use of AgNPs and low-frequency US made it possible to reduce the time of necrosis rejection by 6.5 days (p < 0.05), and the appearance of granulations and epithelial tissue by 3.7 and 4.7 days (p < 0.05), respectively. Simultaneous treatment by the AgNPs with low-frequency US of the purulent CNW showed accelerative tissue regeneration processes, compared to separate AgNPs or US and chlorhexidine treatment methods. A synchronous application of AgNPs with US treatment significantly improves chronic purulent wound healing and reduces the treatment period. The effectiveness of the treatment method is achieved mainly due to the bactericidal properties of AgNPs and the cleansing effect of ultrasonic cavitation.








Similar content being viewed by others
Data Availability
Data sets generated during the current study are available from the corresponding author Myronov PF on reasonable request.
References
Bitsch, M., Laursen, I., Engel, A. M., Christiansen, M., Larsen, S. O., Iversen, L., et al. (2009). Epidemiology of chronic wound patients and relation to serum levels of mannan-binding lectin. Acta Dermato-Venereologica, 89(6), 607–611.
Martinengo, L., Olsson, M., Bajpai, R., Soljak, M., Upton, Z., Schmidtchen, A., et al. (2019). Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Annals of Epidemiology, 29, 8–15.
Rahim, K., Saleha, S., Zhu, X., Huo, L., Basit, A., & Franco, O. L. (2017). Bacterial contribution in chronicity of wounds. Microbial Ecology, 73(3), 710–721.
Malone, M., Bjarnsholt, T., McBain, A. J., James, G. A., Stoodley, P., Leaper, D., et al. (2017). The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. Journal of Wound Care, 26(1), 20–25.
Serra, R., Grande, R., Butrico, L., Rossi, A., Settimio, U. F., Caroleo, B., et al. (2015). Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Review of Anti-Infective Therapy, 13(5), 605–613.
Dilhari, A., Weerasekera, M., Gunasekara, C., Pathirage, S., Fernando, N., Weerasekara, D., et al. (2021). Biofilm prevalence and microbial characterisation in chronic wounds in a Sri Lankan cohort. Letters in Applied Microbiology, 73(4), 477–485.
Buch, P. J., Chai, Y., & Goluch, E. D. (2019). Treating polymicrobial infections in chronic diabetic wounds. Clinical Microbiology Reviews, 32(2), e00091–e00018.
Pang, M., Zhu, M., Lei, X., Xu, P., & Cheng, B. (2019). Microbiome imbalances: an overlooked potential mechanism in chronic nonhealing wounds. The International Journal of Lower Extremity Wounds, 18(1), 31–41.
Tkaczyk, C., Jones-Nelson, O., Shi, Y. Y., Tabor, D. E., Cheng, L., Zhang, T., et al. (2022). Neutralizing Staphylococcus aureus virulence with AZD6389, a three mAb combination, accelerates closure of a diabetic polymicrobial wound. mSphere, 7(3), e0013022.
Versey, Z., da Cruz Nizer, W. S., Russell, E., Zigic, S., DeZeeuw, K. G., Marek, J. E., Overhage, J., & Cassol, E. (2021). Biofilm-innate immune interface: Contribution to chronic wound formation. Frontiers in Immunology, 12, 648554.
Schultz, G., Bjarnsholt, T., James, G. A., Leaper, D. J., McBain, A. J., Malone, M., et al. (2017). Global Wound Biofilm Expert Panel. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair and Regeneration, 25(5), 744–757.
Zhao, R., Liang, H., Clarke, E., Jackson, C., & Xue, M. (2016). Inflammation in chronic wounds. International Journal of Molecular Sciences, 17(12), 2085.
Rabin, N., Zheng, Y., Opoku-Temeng, C., Du, Y., Bonsu, E., & Sintim, H. O. (2015). Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Medicinal Chemistry, 7(4), 493–512.
Goldberg, S. R., & Diegelmann, R. F. (2020). What makes wounds chronic. The Surgical Clinics of North America, 100(4), 681–693.
Wu, Y. K., Cheng, N. C., & Cheng, C. M. (2019). Biofilms in chronic wounds: Pathogenesis and diagnosis. Trends in Biotechnology, 37(5), 505–517.
Azevedo, M. M., Lisboa, C., Cobrado, L., Pina-Vaz, C., & Rodrigues, A. (2020). Hard-to-heal wounds, biofilm and wound healing: an intricate interrelationship. The British Journal of Nursing, 29(5), S6–S13.
Alhede, M., Kragh, K. N., Qvortrup, K., Allesen-Holm, M., van Gennip, M., Christensen, L. D., et al. (2011). Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS One, 6(11), e27943.
Haalboom, M. (2018). Chronic wounds: Innovations in diagnostics and therapeutics. Current Medicinal Chemistry, 25(41), 5772–5781.
Hughes, G., & Webber, M. A. (2017). Novel approaches to the treatment of bacterial biofilm infections. British Journal of Pharmacology, 174(14), 2237–2246.
Chang, Y. R., Perry, J., & Cross, K. (2017). Low-frequency ultrasound debridement in chronic wound healing: A systematic review of current evidence. Plastic Surgery, 25(1), 21–26.
Campitiello, F., Mancone, M., Corte, A. D., Guerniero, R., & Canonico, S. (2018). An evaluation of an ultrasonic debridement system in patients with diabetic foot ulcers: A case series. Journal of Wound Care, 27(4), 222–228.
Volpe, P., Marcuccio, D., Stilo, G., Alberti, A., Foti, G., Volpe, A., et al. (2017). Efficacy of cord blood platelet gel application for enhancing diabetic foot ulcer healing after lower limb revascularization. Seminars in Vascular Surgery, 30(4), 106–112.
Vallejo, A., Wallis, M., McMillan, D., & Horton, E. (2021). Use of low-frequency contact ultrasonic debridement with and without polyhexamethylene biguanide in hard-to-heal leg ulcers: An RCT protocol. Journal of Wound Care, 30(5), 372–379.
Driver, V. R., Yao, M., & Miller, C. J. (2011). Noncontact low-frequency ultrasound therapy in the treatment of chronic wounds: A meta-analysis. Wound Repair and Regeneration, 19(4), 475–480.
Yu, H., Liu, Y., Li, L., Guo, Y., Xie, Y., Cheng, Y., et al. (2020). Ultrasound-involved emerging strategies for controlling foodborne microbial biofilms. Trends in Food Science and Technology, 96, 91–101.
Ojha, K. S., Burgess, C. M., Duffy, G., Kerry, J. P., & Tiwari, B. K. (2018). Integrated phenotypic-genotypic approach to understand the influence of ultrasound on metabolic response of Lactobacillus sakei. PLoS One, 13(1), e0191053.
Dong, Y., Xu, Y., Li, P., Wang, C., Cao, Y., & Yu, J. (2017). Antibiofilm effect of ultrasound combined with microbubbles against Staphylococcus epidermidis biofilm. International Journal of Medical Microbiology, 307(6), 321–328.
Wang, T., Ma, W., Jiang, Z., & Bi, L. (2020). The penetration effect of HMME-mediated low-frequency and low-intensity ultrasound against the Staphylococcus aureus bacterial biofilm. European Journal of Medical Research, 25(1), 51.
Terzi, H. A., Kulah, C., & Ciftci, I. H. (2014). The effects of active efflux pumps on antibiotic resistance in Pseudomonas aeruginosa. World Journal of Microbiology and Biotechnology, 30, 2681–2687.
Cai, Y., Wang, J., Liu, X., Wang, R., & Xia, L. (2017). A review of the combination therapy of low frequency ultrasound with antibiotics. BioMed Research International, 2017, 2317846.
Liu, X., Wang, J., Weng, C. X., Wang, R., & Cai, Y. (2020). Low-frequency ultrasound enhances bactericidal activity of antimicrobial agents against Klebsiella pneumoniae biofilm. BioMed Research International, 2020, 5916260.
Seth, A. K., Nguyen, K. T., Geringer, M. R., Hong, S. J., Leung, K. P., Mustoe, T. A., et al. (2013). Noncontact, low-frequency ultrasound as an effective therapy against Pseudomonas aeruginosa-infected biofilm wounds. Wound Repair and Regeneration, 21(2), 266–274.
Pitt, W. G., & Ross, S. A. (2003). Ultrasound increases the rate of bacterial cell growth. Biotechnology Progress, 19(3), 1038–1044.
Wang, J., Wen, K., Liu, X., Weng, C. X., Wang, R., & Cai, Y. (2018). Multiple low frequency ultrasound enhances bactericidal activity of vancomycin against methicillin-resistant Staphylococcus aureus biofilms. BioMed Research International, 2018, 6023101.
Liu, X., Yin, H., Weng, C. X., & Cai, Y. (2016). Low-frequency ultrasound enhances antimicrobial activity of colistin-vancomycin combination against pan-resistant biofilm of acinetobacter baumannii. Ultrasound in Medicine & Biology, 42(8), 1968–1975.
Lee, S. H., & Jun, B. H. (2019). Silver nanoparticles: Synthesis and application for nanomedicine. International Journal of Molecular Sciences, 20(4), 865.
Szczepanowicz, K., Stefańska, J., Socha, R. P., & Warszyński, P. (2010). Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity. Physicochemical Problems of Mineral Processing, 45, 85–98.
Deshmukh, S. P., Patil, S. M., Mullani, S. B., & Delekar, S. D. (2019). Silver nanoparticles as an effective disinfectant: A review. Materials Science & Engineering C Materials for Biological Applications, 97, 954–965.
Guo, J., Qin, S., Wei, Y., Liu, S., Peng, H., Li, Q., et al. (2019). Silver nanoparticles exert concentration-dependent influences on biofilm development and architecture. Cell Proliferation, 52(4), e12616.
Estevez, M. B., Raffaelli, S., Mitchell, S. G., Faccio, R., & Alborés, S. (2020). Biofilm eradication using biogenic silver nanoparticles. Molecules, 25(9), 2023.
Zhang, C., Hu, Z., & Deng, B. (2016). Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms. Water Research, 88, 403–427.
Yuan, B., Sui, M., Lu, H., Wang, J., & Qin, J. (2019). The combined effect of light irradiation and chloride on the physicochemical properties of silver nanoparticles. RSC Advances, 10(1), 228–235.
Zou, X., Li, P., Lou, J., Fu, X., & Zhang, H. (2017). Stability of single dispersed silver nanoparticles in natural and synthetic freshwaters: Effects of dissolved oxygen. Environmental Pollution, 230, 674–682.
Bélteky, P., Rónavári, A., Zakupszky, D., Boka, E., Igaz, N., Szerencsés, B., et al. (2021). Are smaller nanoparticles always better? Understanding the biological effect of size-dependent silver nanoparticle aggregation under biorelevant conditions. International Journal of Nanomedicine, 16, 3021–3040.
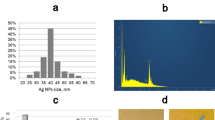
Myronov, P., Bugaiov, V., Holubnycha, V., Sikora, V., Deineka, V., Lyndin, M., et al. (2020). Low-frequency ultrasound increase effectiveness of silver nanoparticles in a purulent wound model. Biomedical Engineering Letters, 10(4), 621–631.
Holubnycha V, Myronov P, Bugaiov V, Opanasyuk A, Dobrozhan O, Yanovska A, et al. Effect of ultrasound treatment on chitosan-silver nanoparticles antimicrobial activity. Conf Proc IEEE 8th International Conference on Nanomaterials: Applications and Properties. 2018; 04NNLS09-1–04NNLS09-4.
Wang, P. H., Huang, B. S., Horng, H. C., Yeh, C. C., & Chen, Y. J. (2018). Wound healing. Journal of the Chinese Medical Association, 81(2), 94–101.
Bowers, S., & Franco, E. (2020). Chronic wounds: Evaluation and management. American Family Physician, 101(3), 159–166.
Krishnaswamy, V. R., Mintz, D., & Sagi, I. (2017). Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1864(11 Pt B), 2220–2227. https://doi.org/10.1016/j.bbamcr.2017.08.003
Sorg, H., Tilkorn, D. J., Hager, S., Hauser, J., & Mirastschijski, U. (2017). Skin wound healing: An update on the current knowledge and concepts. European Surgical Research, 58(1-2), 81–94.
Tashi, T., Vishal, G. N., & Mbuya, V. B. (2016). Silver nanoparticles: Synthesis, mechanism of antimicrobial action, characterization, medical applications, and toxicity effects. Journal of Chemical and Pharmaceutical Research, 8(2), 526–537.
Anh, N. P., Linh, D. N., Minh, N. V., & Tri, N. (2019). Positive effects of the ultrasound on biosynthesis, characteristics and antibacterial activity of silver nanoparticles using Fortunella japonica. Materials Transactions, 60(9), 2053–2058.
Polat, B. E., Blankschtein, D., & Langer, R. (2010). Low-frequency sonophoresis: Application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opinion on Drug Delivery, 7(12), 1415–1432.
Tian, J., Wong, K. K. Y., Ho, C. M., Lok, C. N., Yu, W. Y., Che, C. M., Chiu, J. F., & Tam, P. K. H. (2007). Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem, 2(1), 129–136.
Acknowledgements
We highly appreciate the support given by Dr. Sukhvinder Sandhu who provided English proof-reading and corrections.
Funding
This research was supported by Horizon-Europe MSCA-SE project ARGO (№ 101086441).
Author information
Authors and Affiliations
Contributions
P.M. proposed the idea, formulated overarching research goals and aims, and conducted an experiment on laboratory animals. I.D. developed the design and the main stages of the experiment: prepared and wrote the initial draft of the published work (including substantive translation); and reviewed and approved the final version of the manuscript. V.B. proposed an initial hypothesis for the successful combination of silver nanoparticles and low-frequency ultrasound in the treatment of chronic purulent wounds; developed and tested a model of a chronic purulent wound on laboratory animals; and wrote a draft of the literature review. V.H. prepared a mixture of microorganisms for the successful modeling of a chronic purulent wound; prepared pictures 1, 2, 3, and 8; and made a critical review, commentary, and revision of the manuscript. V.S. took histological material and conducted histological studies, calculated skin morphometric parameters, and prepared pictures 4, 5, 6, and 7. A.O. synthesized silver nanoparticles and prepared them in the required concentrations; conducted part of the experiment on laboratory animals using low-frequency ultrasound; and made suggestions on the practical application of low-frequency ultrasound in laboratory animals. A.R. conducted an analysis of histological studies; reviewed and edited the manuscript; and proposed and substantiated the repeated experiment in groups using silver nanoparticles for a more detailed histological analysis. O.P. conducted a statistical analysis of the studies using GraphPad Prism 8.0 software; and ensured the maintenance of animals in the vivarium and compliance with ethical standards during the experiment (including general anesthesia of rats). M.P. was responsible for the management and coordination of the research activity planning and execution; provided financial support for the project leading to this publication; and reviewed and approved the final version of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
Keeping, feeding, handling of animals, and all experiments were carried out in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines 2.0. and with Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. The Bioethics Committee of Experimental and Clinical Research of Sumy State University approved the experimental protocols (№ 4/4, 15.04.2019; № 2/9, 17.09.2021).
Informed Consent
The research did not involve human subjects or personal information. No informed consent is required.
Ethics Approval
All rat handling and experimental procedures fully adhere to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines 2.0. and European Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. The Bioethics Committee of Experimental and Clinical Research of Sumy State University approved the experimental protocols (№ 4/4, 15.04.2019; № 2/9, 17.09.2021).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Myronov, P., Duzhyi, I., Bugaiov, V. et al. Low-Frequency Ultrasound Reinforces Silver Nanoparticles Effect in Experimental Chronic Non-healing Purulent Wounds Treatment. BioNanoSci. 13, 2337–2347 (2023). https://doi.org/10.1007/s12668-023-01195-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01195-x