Abstract
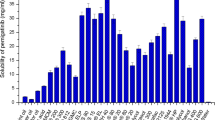
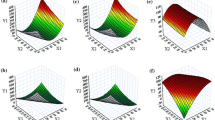
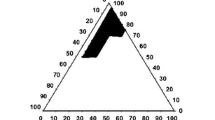
Entrectinib is a novel potent anticancer drug with poor aqueous solubility. A supersaturable self-nanoemulsifying drug delivery system of entrectinib is developed using a super saturation promoter. The components of the isotropic mixture of SNEDDS were selected based on solubility and emulsification study. The optimum composition was identified using phase diagrams and further optimized by mixture design. The supersaturated SNEDDS was prepared using HPMC K4M as precipitation inhibitor. The droplet of sSNEDDS ranges from 118.42 ± 1.26 to 128.34 ± 0.63 nm with PDI values range from 0.112 to 0.204, which is significantly smaller than that observed with plain SNEDDS. The percent transmittance of the diluted formulation was found to be 98.78 ± 0.74. The viscosity was found to be 528 ± 32 centipoises indicating the good flow ability. FTIR and DSC studies indicated the amorphization of the drug. The dissolution profile of sSNEDDS indicated the faster release of drug compared to both pure drug suspension and SNEDDS formulation. The drug release rate is directly proportional to the concentration of the drug. The drug release from the insoluble matrix is a square root of time-dependent Fickian diffusion process. The formulation was found to be stable and transparent at all pH values and the percent transmittance was more than 95%. No significant difference was observed with all the samples exposed at different storage conditions. This study demonstrated the feasibility of stabilizing and improving the in vitro performance of SNEDDS by incorporating HPMC K4M as precipitation inhibitor.
Graphical abstract














Similar content being viewed by others
Abbreviations
- NBDDS:
-
nano-based drug delivery system
- SNEDDS:
-
self-nanoemulsifying drug delivery system
- sSNEDDS:
-
supersaturable self-nanoemulsifying drug delivery system
- PDI:
-
polydispersity index
- HPMC:
-
hydroxy propyl methyl cellulose
- FTIR spectroscopy:
-
Fourier transformed infrared spectroscopy
- DSC:
-
differential scanning calorimetry
- TEM:
-
transmission electron microscope
- FGFR:
-
fibroblast growth factor receptor
- US FDA:
-
United States Food and Drug Administration
- BCS:
-
biopharmaceutical classification systems
- GRAS:
-
generally recognized as safe
- GIT:
-
gastro intestinal tract
References
Weinstein, I. B., & Joe, A. K. (2006). Mechanisms of disease: Oncogene addiction–A rationale for molecular targeting in cancer therapy. Nature Clinical Practice. Oncology, 3(8), 448–457. https://doi.org/10.1038/ncponc0558
Frampton, J. E. (2021). Entrectinib: A review in NTRK+ solid tumours and ROS1+ NSCLC. Drugs, 81, 697–708. https://doi.org/10.1007/s40265-021-01503-3
Rolfo, C., Ruiz, R., Giovannetti, E., Gil-Bazo, I., Russo, A., Passiglia, F., Giallombardo, M., Peeters, M., & Raez, L. (2015). Entrectinib: A potent new TRK, ROS1, and ALK inhibitor. Expert Opinion on Investigational Drugs, 24(11), 1493–1500. https://doi.org/10.1517/13543784.2015.1096344
Delgado, J., Pean, E., Melchiorri, D., Migali, C., Josephson, F., Enzmann, H., & Pignatti, F. (2021). The European Medicines Agency review of entrectinib for the treatment of adult or paediatric patients with solid tumours who have a neurotrophic tyrosine receptor kinase gene fusions and adult patients with non-small-cell lung cancer harbouring ROS1 rearrangements. ESMO Open, 6(2), 100087. https://doi.org/10.1016/j.esmoop.2021.100087
Sigal, D., Tartar, M., Xavier, M., Bao, F., Foley, P., Luo, D., Christiansen, J., Hornby, Z., Maneval, E. C., & Multani, P. (2017). Activity of entrectinib in a patient with the first reported NTRK fusion in neuroendocrine cancer. Journal of the National Comprehensive Cancer Network, 15, 1317–1322. https://doi.org/10.6004/jnccn.2017.7029
Meneses-Lorente, G., Bentley, D., Guerini, E., Kowalski, K., Chow-Maneval, E., Yu, L., Brink, A., Djebli, N., Mercier, F., Buchheit, V., & Phipps, A. B. (2021). Characterization of the pharmacokinetics of entrectinib and its active M5 metabolite in healthy volunteers and patients with solid tumors. Investigational New Drugs, 39(3), 803–811. https://doi.org/10.1007/s10637-020-01047-5
Al-Salama, Z. T., & Keam, S. J. (2019). Entrectinib: First global approval. Drugs, 79(13), 1477–1483. https://doi.org/10.1007/s40265-019-01177-y
Parrott, N., Stillhart, C., Lindenberg, M., Wagner, B., Kowalski, K., Guerini, E., Djebli, N., & Meneses-Lorente, G. (2020). Physiologically based absorption modelling to explore the impact of food and gastric pH changes on the pharmacokinetics of entrectinib. The AAPS Journal, 22(4), 78. https://doi.org/10.1208/s12248-020-00463-y
Sharma, M., Sharma, R., & Jain, D. K. (2016). Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Scientifica. https://doi.org/10.1155/2016/8525679
Morakul, B. (2020). Self-nanoemulsifying drug delivery systems (SNEDDS): An advancement technology for oral drug delivery. Pharm Sci Asia, 47(3), 205–220. https://doi.org/10.1208/s12249-020-01765-2
Salawi, A. (2022). Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Delivery, 29(1), 1811–1823. https://doi.org/10.1080/10717544.2022.2083724
Mishra, D. K., Shandilya, R., & Mishra, P. K. (2018). Lipid based nanocarriers: A translational perspective. Nanomedicine: Nanotechnology, Biology and Medicine, 14(7), 2023–2050. https://doi.org/10.1016/j.nano.2018.05.021
Shrestha, H., Bala, R., & Arora, S. (2014). Lipid-based drug delivery systems. Journal of Pharmaceutics. https://doi.org/10.1155/2014/801820
Krstić, M., Medarević, Đ., Đuriš, J., & Ibrić, S. (2018). Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In M. G. Alexandru (Ed.), Lipid nanocarriers for drug targeting (pp. 473–508). William Andrew Publishing. https://doi.org/10.1016/B978-0-12-813687-4.00012-8
Wang, C. Y., Yen, C. C., Hsu, M. C., & Wu, Y. T. (2020). Self-nanoemulsifying drug delivery systems for enhancing solubility, permeability, and bioavailability of sesamin. Molecules, 25(14), 3119. https://doi.org/10.3390/molecules25143119
Patel, J., Patel, A., Raval, M., & Sheth, N. (2011). Formulation and development of a self-nanoemulsifying drug delivery system of irbesartan. Journal of Advanced Pharmaceutical Technology & Research, 2(1), 9–16 https://www.japtr.org/text.asp?2011/2/1/9/79799
Anuar, N., Sabri, A. H., Effendi, T. J. B., & Hamid, K. A. (2020). Development and characterisation of ibuprofen-loaded nanoemulsion with enhanced oral bioavailability. Heliyon, 6(7), e04570. https://doi.org/10.1016/j.heliyon.2020.e04570
Ahmed, O. A., Badr-Eldin, S. M., Tawfik, M. K., Ahmed, T. A., Khalid, M., & Badr, J. M. (2014). Design and optimization of self-nanoemulsifying delivery system to enhance quercetin hepatoprotective activity in paracetamol-induced hepatotoxicity. Journal of Pharmaceutical Sciences, 103(2), 602–612. https://doi.org/10.1002/jps.23834
Zhang, J., Wen, X., Dai, Y., & Xia, Y. (2019). Mechanistic studies on the absorption enhancement of a self-nanoemulsifying drug delivery system loaded with norisoboldine-phospholipid complex. International Journal of Nanomedicine, 14, 7095–7106. https://doi.org/10.2147/IJN.S211905
Buya, A. B., Beloqui, A., Memvanga, P. B., & Préat, V. (2020). Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics, 12(12), 1194. https://doi.org/10.3390/pharmaceutics12121194
Thomas, N., Müllertz, A., Graf, A., & Rades, T. (2012). Influence of lipid composition and drug load on the in vitro performance of self-nanoemulsifying drug delivery systems. Journal of Pharmaceutical Sciences, 101(5), 1721–1731. https://doi.org/10.1002/jps.23054
Bandyopadhyay, S., Katare, O. P., & Singh, B. (2014). Development of optimized supersaturable self-nanoemulsifying systems of ezetimibe: Effect of polymers and efflux transporters. Expert Opinion on Drug Delivery, 11(4), 479–492. https://doi.org/10.1517/17425247.2014.877885
Nazlı, H., Mesut, B., & Özsoy, Y. (2021). In vitro evaluation of a solid supersaturated self nanoemulsifying drug delivery system (Super-SNEDDS) of aprepitant for enhanced solubility. Pharmaceuticals, 14(11), 1089. https://doi.org/10.3390/ph14111089
Thomas, N., Holm, R., Müllertz, A., & Rades, T. (2012). In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). Journal of Controlled Release, 160(1), 25–32. https://doi.org/10.1016/j.jconrel.2012.02.027
Nair, A. B., Singh, B., Shah, J., Jacob, S., Aldhubiab, B., Sreeharsha, N., Morsy, M. A., Venugopala, K. N., Attimarad, M., & Shinu, P. (2022). Formulation and evaluation of self-nanoemulsifying drug delivery system derived tablet containing sertraline. Pharmaceutics, 14(2), 336. https://doi.org/10.3390/pharmaceutics14020336
Nasr, A., Gardouh, A., & Ghorab, M. (2016). Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: Design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics, 8(3), 20. https://doi.org/10.3390/pharmaceutics8030020
Shastri, P. N., Ubale, R. V., & D’Souza, M. J. (2013). Implementation of mixture design for formulation of albumin containing enteric-coated spray-dried microparticles. Drug Development and Industrial Pharmacy, 39(2), 164–175. https://doi.org/10.3109/03639045.2012.664148
Patel, M., Shaikh, F., & Surti, N. (2021). Optimization of glipizide floating matrix tablet using simplex lattice design. Indian Journal of Pharmaceutical Sciences, 83(2), 297–306. https://doi.org/10.36468/pharmaceutical-sciences.776
Sanka, K., Suda, D., & Bakshi, V. (2016). Optimization of solid-self nanoemulsifying drug delivery system for solubility and release profile of clonazepam using simplex lattice design. Journal of Drug Delivery Science and Technology, 33, 114–124. https://doi.org/10.1016/j.jddst.2016.04.003
Indrati, O., Martien, R., Rohman, A., & Nugroho, A. K. (2020). Application of simplex lattice design on the optimization of andrographolide self-nanoemulsifying drug delivery system (SNEDDS). Indones J Pharm, 31(2), 124–130. https://doi.org/10.14499/indonesianjpharm31iss2pp124
Rangaraj, N., Shah, S., Maruthi, A. J., Pailla, S. R., Cheruvu, H. S., Sujatha, D., & Sampathi, S. (2019). Quality by design approach for the development of self-emulsifying systems for oral delivery of febuxostat: Pharmacokinetic and pharmacodynamic evaluation. AAPS PharmSciTech, 20(7), 1–16. https://doi.org/10.1208/s12249-019-1476-y
Czajkowska-Kośnik, A., Szekalska, M., Amelian, A., Szymańska, E., & Winnicka, K. (2015). Development and evaluation of liquid and solid self-emulsifying drug delivery systems for atorvastatin. Molecules, 20(12), 21010–21022. https://doi.org/10.3390/molecules201219745
Wu, L., Qiao, Y., Wang, L., Guo, J., Wang, G., He, W., Yin, L., & Zhao, J. (2015). A self-microemulsifying drug delivery system (SMEDDS) for a novel medicative compound against depression: A preparation and bioavailability study in rats. AAPS PharmSciTech, 16(5), 1051–1058. https://doi.org/10.1208/s12249-014-0280-y
Kazi, M., Al-Swairi, M., Ahmad, A., Raish, M., Alanazi, F. K., Badran, M. M., Khan, A. A., Alanazi, A. M., & Hussain, M. D. (2019). Evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for poorly water-soluble talinolol: Preparation, in vitro and in vivo assessment. Frontiers in Pharmacology, 10, 459. https://doi.org/10.3389/fphar.2019.00459
Beg, S., Katare, O. P., & Singh, B. (2017). Formulation by design approach for development of ultrafine self-nanoemulsifying systems of rosuvastatin calcium containing long-chain lipophiles for hyperlipidemia management. Colloids and Surfaces. B, Biointerfaces, 159, 869–879. https://doi.org/10.1016/j.colsurfb.2017.08.050
Weerapol, Y., Limmatvapirat, S., Nunthanid, J., & Sriamornsak, P. (2014). Self-nanoemulsifying drug delivery system of nifedipine: Impact of hydrophilic–lipophilic balance and molecular structure of mixed surfactants. AAPS PharmSciTech, 15(2), 456–464. https://doi.org/10.1208/s12249-014-0078-y
Availability of Data and Material
Will be made available on request.
Author information
Authors and Affiliations
Contributions
MRR carried out the entire research and prepared the manuscript. KSG guided the work and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable as no animal or humans are used in this study.
Consent for Publication
This work is original and not published or under consideration in any other journal.
Conflict of Interest
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reddy, M.R., Gubbiyappa, K.S. Formulation Development, Optimization, and Characterization of Entrectinib-Loaded Supersaturable Self-Nanoemulsifying Drug Delivery Systems. BioNanoSci. 13, 521–540 (2023). https://doi.org/10.1007/s12668-023-01094-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01094-1