Abstract
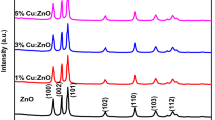
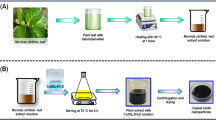
Zinc oxide (ZnO) and Cu-doped ZnO nanoparticles (1% and 5%; Cu-doped ZnO NPs) were synthesized using aqueous leaf extract of Stachytarpheta jamaicensis. Crystalline ZnO and Cu-doped ZnO NPs with hexagonal wurtzite structure were obtained without any impurities as confirmed by powder X-ray diffraction. The optical properties, morphology, elemental composition, and surface analysis of all the samples were studied using UV-vis diffuse reflectance spectroscopy, scanning electron microscopy equipped with energy dispersive X-ray analysis (SEM-EDX), and X-ray photoelectron spectroscopy, respectively. Antibacterial activities of the ZnO and Cu-doped ZnO were performed by screening test at the highest concentration (500 mg/mL) against two gram-positive bacterial strains (Bacillus subtilis and Staphylococcus aureus) and two gram-negative bacterial strains (Pseudomonas aeruginosa and Escherichia coli). Antibacterial activities were observed only against gram-positive bacteria. Minimum inhibitory concentration (MIC) ranged from < 25 to 50 mg/mL.










Similar content being viewed by others
References
Ansari, S. A., Khan, M. M., Kalathil, S., Nisar, A., Lee, J., & Cho, M. H. (2013). Oxygen vacancy induced band gap narrowing of ZnO nanostructure by electrochemically active biofilm. Nanoscale, 5, 9238–9246. https://doi.org/10.1039/c3nr02678g.
Kennedy, J., Murmu, P. P., Manikandan, E., & Lee, S. Y. (2014). Investigation of structural and photoluminescence properties of gas and metal ions doped zinc oxide single crystals. Journal of Alloys and Compounds, 616, 614–617. https://doi.org/10.1016/j.jallcom.2014.07.179.
Khan, M. M., Saadah, N. H., Khan, M. E., Harunsani, M. H., Tan, A. L., & Cho, M. H. (2019). Phytogenic synthesis of band gap-narrowed ZnO nanoparticles using the bulb extract of Costus woodsonii. Bionanoscience, 9, 334–344. https://doi.org/10.1007/s12668-019-00616-0.
Khan, M. M., Saadah, N. H., Khan, M. E., Harunsani, M. H., Tan, A. L., & Cho, M. H. (2019). Potentials of Costus woodsonii leaf extract in producing narrow band gap ZnO nanoparticles. Materials Science in Semiconductor Processing, 91, 194–200. https://doi.org/10.1016/j.mssp.2018.11.030.
Makarov, V. V., Love, A. J., Sinitsyna, O. V., Makarova, S. S., Yaminsky, I. V., Taliansky, M. E., & Kalinina, N. O. (2014). “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae, 6, 35–44 https://www.ncbi.nlm.nih.gov/pubmed/24772325.
Yuvakkumar, R., Suresh, J., & Hong, S. I. (2014). Green synthesis of zinc oxide nanoparticles. Advances in Materials Research, 952, 137–140. https://doi.org/10.4028/www.scientific.net/AMR.952.137.
Gunalan, S., Sivaraj, R., & Rajendran, V. (2012). Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Progress in Natural Science: Materials International, 22, 693–700. https://doi.org/10.1016/j.pnsc.2012.11.015.
Happy, A., Menon, S., Venkat Kumar, S., & Rajeshkumar, S. (2018). Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chemico-Biological Interactions, 286, 60–70. https://doi.org/10.1016/j.cbi.2018.03.008.
Černík, M., Thekkae Padil, V.V. (2013). Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application, International Journal of Nanomedicine 889. doi:https://doi.org/10.2147/IJN.S40599.
Ajitha, B., Kumar Reddy, Y. A., Reddy, P. S., Jeon, H. J., & Ahn, C. W. (2016). Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Advances, 6, 36171–36179. https://doi.org/10.1039/c6ra03766f.
Liew, P.M., Yong, Y.K. (2016). Stachytarpheta jamaicensis (L.) Vahl: from traditional usage to pharmacological evidence, evidence-based complement. Evidence-Based Complementary and Alternative Medicine 2016. doi:https://doi.org/10.1155/2016/7842340.
Ezenwa, K. C., Ighodaro, I., & Macdonald, I. (2015). Antidiabetic activity of methanol extract of stachytarpheta jamaicensis in streptozotocin-induced diabetic rats. Nigerian Journal of Pharmaceutical Sciences, 14, 9–18.
Pandian, C., Srinivasan, A., & Pelapolu, I. C. (2013). Evaluation of wound healing activity of hydroalcoholic extract of leaves of Stachytarpheta jamaicensis in streptozotocin induced diabetic rats. Der Pharmacia Lettre., 5, 193–200.
Putera, K.A.S.I., Hamdan, S., Dan Biokejuruteraan, F.B. (2010). Antimicrobial activity and cytotoxic effects of Stachytarpheta jamaicensis (L), Vahl Crude Plant Extracts, Universiti Teknologi Malaysia. https://books.google.com.bn/books?id=7j9nnQAACAAJ.
Murugan, M., Murugan, T., & Wins, J. A. (2013). Antimicrobial activity and phytochemical constituents of leaf extracts of Cassia auriculata. Indian Journal of Pharmaceutical Sciences, 75, 122. https://doi.org/10.4103/0250-474X.113546.
Mitchell, G. J., Wiesenfeld, K., Nelson, D. C., & Weitz, J. S. (2013). Critical cell wall hole size for lysis in gram-positive bacteria. Journal of The Royal Society Interface, 10, 20120892. https://doi.org/10.1098/rsif.2012.0892.
Dizaj, S. M., Lotfipour, F., Barzegar-Jalali, M., Zarrintan, M. H., & Adibkia, K. (2014). Antimicrobial activity of the metals and metal oxide nanoparticles. Materials Science and Engineering: C, 44, 278–284. https://doi.org/10.1016/j.msec.2014.08.031.
Silhavy, T. J., Kahne, D., & Walker, S. (2010). The bacterial cell envelope. Cold Spring Harbor Perspectives in Biology, 2, a000414. https://doi.org/10.1101/cshperspect.a000414.
Shannon, R. D. (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A, 32, 751–767. https://doi.org/10.1107/S0567739476001551.
Banu Bahşi, Z., & Oral, A. Y. (2007). Effects of Mn and Cu doping on the microstructures and optical properties of sol-gel derived ZnO thin films. Optical Materials, 29, 672–678. https://doi.org/10.1016/j.optmat.2005.11.016.
Robak, E., Coy, E., Kotkowiak, M., Jurga, S., Załęski, K., & Drozdowski, H. (2016). The effect of Cu doping on the mechanical and optical properties of zinc oxide nanowires synthesized by hydrothermal route. Nanotechnology, 27, 175706. https://doi.org/10.1088/0957-4484/27/17/175706.
Herng, T. S., Lau, S. P., Yu, S. F., Yang, H. Y., Wang, L., Tanemura, M., & Chen, J. S. (2007). Magnetic anisotropy in the ferromagnetic Cu-doped ZnO nanoneedles. Applied Physics Letters, 90, 032509. https://doi.org/10.1063/1.2433028.
Yugandhar, P., Vasavi, T., Uma Maheswari Devi, P., & Savithramma, N. (2017). Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Applied Nanoscience, 7, 417–427. https://doi.org/10.1007/s13204-017-0584-9.
Shanmugam, V., & Jeyaperumal, K. S. (2018). Investigations of visible light driven Sn and Cu doped ZnO hybrid nanoparticles for photocatalytic performance and antibacterial activity. Applied Surface Science, 449, 617–630. https://doi.org/10.1016/j.apsusc.2017.11.167.
Bauer, A. W., Kirby, W. M., Sherris, J. C., & Turck, M. (1966). Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, 45, 493–496. https://doi.org/10.1093/ajcp/45.4_ts.493.
Gumustas, M., Sengel-Turk, C.T., Gumustas, A., Ozkan, S.A., Uslu, B. (2017). Effect of polymer-based nanoparticles on the assay of antimicrobial drug delivery systems, in: Multifunct. Syst. Comb. Deliv. Biosensing Diagnostics, Elsevier: pp. 67–108. doi:https://doi.org/10.1016/B978-0-323-52725-5.00005-8.
Ren, G., Hu, D., Cheng, E. W., Vargas-Reus, M. A., Reip, P., & Allaker, R. P. (2009). Characterisation of copper oxide NPs for antimicrobial applications. International Journal of Antimicrobial Agents, 33(6), 587–590. https://doi.org/10.1016/j.ijantimicag.2008.12.004.
Oves, M., Arshad, M., Khan, M. S., Ahmed, A. S., Azam, A., & Ismail, I. M. I. (2015). Anti-microbial activity of cobalt doped zinc oxide nanoparticles: Targeting water borne bacteria. Journal of Saudi Chemical Society, 19(5), 581–588. https://doi.org/10.1016/j.jscs.2015.05.003.
Wang, L., Hu, C., & Shao, L. (2017). The antimicrobial activity of nanoparticles: present situation and prospects for the future. International Journal of Nanomedicine, 12, 1227–1249. https://doi.org/10.2147/IJN.S121956.
Khan, M. M., Harunsani, M. H., Tan, A. L., Hojamberdiev, M., Azamay, S., & Ahmad, N. (2020). Antibacterial activities of zinc oxide and Mn-doped zinc oxide synthesized using Melastoma malabathricum (L.) leaf extract. Bioprocess and Biosystems Engineering, 43(8), 1499–1508. https://doi.org/10.1007/s00449-020-02343-3.
Shaikh, S., Nazam, N., Rizvi, S. M. D., Ahmad, K., Baig, M. H., Lee, E. J., & Choi, I. (2019). Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. International Journal of Molecular Sciences, 20(10), 2468. https://doi.org/10.3390/ijms20102468.
Acknowledgments
Authors would like to acknowledge the FIC grant UBD/RSCH/1.4/FICBF(b)/2018/012 from Universiti Brunei Darussalam, Brunei Darussalam.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, M.M., Harunsani, M.H., Tan, A.L. et al. Antibacterial Studies of ZnO and Cu-Doped ZnO Nanoparticles Synthesized Using Aqueous Leaf Extract of Stachytarpheta jamaicensis. BioNanoSci. 10, 1037–1048 (2020). https://doi.org/10.1007/s12668-020-00775-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-020-00775-5