Abstract
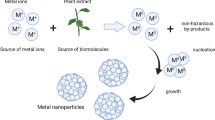
Green chemistry becomes an eye-catching topic of interest in the past few years because it is a comfortable, secure, inexpensive, and eco-friendly way of synthesis. Iron oxide nanoparticles with different morphologies and sizes have been extensively studied due to their broad applications. Iron nanoparticles (Fe NPs) have drawn interest in site remediation and also in the treatment of organic or inorganic pollutants of water. The present review shows different synthesis methods of zero-valent and iron oxide nanoparticles from different plant extracts including tea extracts (Oolong tea, tea powder, tea waste, and tea polyphenols), from other plant extracts (Amaranthus dubius, Murraya koenigii, Eucalyptus, Syzygium aromaticum, curcuma, Ocimum sanctum, Emblica officinalis, Tridax procumbens, Dodonaea viscosa, Spinacia oleracea, Lawsonia inermis (henna), Gardenia jasminoides, Punica granatum, and Colocasia esculenta), from bio-microorganisms (Acinetobacter spp. bacterium, Aspergillus oryzae, Sargassum muticum), and from magnetite sand. The different potential applications of iron nanoparticles in remediation, in dye removal, and as an antibacterial agent point to the importance of iron nanoparticles in the environmental removal of contamination.



Similar content being viewed by others
References
Usepa U. (2007). Nanotechnology White Paper. Prepared for the US Environmental Protection Agency by Members of the Nanotechnology Workgroup, a Group of Epa's Science Policy Council Science Policy Council (pp. 20460). Washington, DC: US Environmental Protection Agency.
Thakkar, K. N., Mhatre, S. S., & Parikh, R. Y. (2010). Biological synthesis of metallic nanoparticles. Nanomedicine, 6, 257–262.
Panigrahi, S., Kundu, S., Ghosh, S. K., Nath, S., & Pal, T. (2004). General method of synthesis for metal nanoparticles. Journal of Nanoparticle Research, 6, 411–414.
T E R I. (2010). Nanotechnology development in India: building capability and governing the technology [TERI Briefing Paper], supported by IDRC, Canada.
Senapati, S., Ahmad, A., Khan, M. I., Sastry, M., & Kumar, R. (2005). Extracellular biosynthesis of bimetallic Au–Ag alloy nanoparticles. Small, 1, 517–520.
Klaus, T., Joerger, R., Olsson, E., & Granqvist, C. G. (1999). Silver-based crystalline nanoparticles, microbially fabricated. Proceedings of the National Academy of Sciences of the United States of America, 96, 13611–13614.
Rai, M., Yadav, A., & Gade, A. (2008). Current trends in phytosynthesis of metal nanoparticles. Critical Reviews in Biotechnology, 28, 277–284.
Mohanpuria, P., Rana, N. K., & Yadav, S. K. (2008). Biosynthesis of nanoparticles: technological concepts and future applications. Journal of Nanoparticle Research, 10, 507–517.
Pankhurst, Q. A., Connolly, J., Jones, S. K., & Dobson, J. (2003). Applications of magnetic nanoparticles in biomedicine. Journal of Physics D, 36, 167–181.
Gilchrist, R. K., Medal, R., Shorey, W. D., Hanselman, R. C., Parrot, J. C., & Taylor, C. B. (1957). Selective inductive heating of lymph nodes. Annals of Surgery, 146, 596–606.
Zboril, R., Mashlan, M., & Petridis, D. (2002). Iron(III) oxides from thermal processes—synthesis, structural and magnetic properties, Mössbauer spectroscopy characterization, and applications. Chemistry of Materials, 14, 969–982.
Shin, E. J., Miser, D. E., Chan, W. G., & Hajaligol, M. R. (2005). Catalytic cracking of catechols and hydroquinones in the presence of nano-particle iron oxide. Applied Catalysis, 61, 79–89.
Prucek, R., Hermanek, M., & Zbořil, R. (2009). An effect of iron(III) oxides crystallinity on their catalytic efficiency and applicability in phenol degradation—a competition between homogeneous and heterogeneous catalysis. Applied Catalysis, 366, 325–232.
Rettig, F., & Moos, R. (2010). α-Iron oxide: an intrinsically semiconducting oxide material for direct thermoelectric oxygen sensors. Sensors and Actuators B: Chemical, 145, 685–690.
Cesar, I., Kay, A., Martinez, J. A. G., & Grätzel, M. (2006). Translucent thin film Fe2O3 photoanodes for efficient water splitting by sunlight: nanostructure-directing effect of Si-doping. Journal of the American Chemical Society, 128, 4582–4583.
Amara, D., Grinblat, J., & Margel, S. (2012). Solventless thermal decomposition of ferrocene as a new approach for one-step synthesis of magnetite nanocubes and nanospheres. Journal of Materials Chemistry, 22, 2188–2195.
Breen, M. L., Dinsmore, A. D., Pink, R. H., Qadri, S. B., & Ratna, B. R. (2001). Sonochemically produced ZnS-coated polystyrene core−shell particles for use in photonic crystals. Langmuir, 17, 903–907.
Deng, Y., Qi, D., Deng, C., Zhang, X., & Zhao, D. (2008). Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. Journal of the American Chemical Society, 130, 28–29.
Caruso, F., Susha, A. S., Giersig, M., & Möhwald, H. (1999). Magnetic core–shell particles: preparation of magnetite multilayers on polymer latex microspheres. Advanced Materials, 11, 950–953.
Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Elst, L. V., & Muller, R. N. (2008). Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chemical Reviews, 108, 2064–2110.
Bulte, J. W., Douglas, T., Witwer, B., Zhang, S. C., Strable, E., Lewis, B. K., Zywicke, H., Miller, B., van Gelderen, P., Moskowitz, B. M., & Duncan, I. D. (2001). Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nature Biotechnology, 19(12), 1141.
Arbab, A. S., Yocum, G. T., Kalish, H., Jordan, E. K., Anderson, S. A., Khakoo, A. Y., Read, E. J., & Frank, J. A. (2004). Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood, 104(4), 1217–1223.
Kalish, H., Arbab, A. S., Miller, B. R., Lewis, B. K., Zywicke, H. A., Bulte, J. W., Bryant, L. H., & Frank, J. A. (2003). Combination of transfection agents and magnetic resonance contrast agents for cellular imaging: relationship between relaxivities, electrostatic forces, and chemical composition. Magnetic Resonance in Medicine, 50(2), 275–282.
Schulze, E., Ferrucci, J. J., Poss, K., Lapointe, L., Bogdanova, A., & Weissleder, R. (1995). Cellular uptake and trafficking of a prototypical magnetic iron oxide label in vitro. Investigative Radiology, 30(10), 604–610.
Moore, A., Marecos, E., Bogdanov Jr., A., & Weissleder, R. (2000). Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology, 214(2), 568–574.
Sipe, J. C., Filippi, M., Martino, G., Furlan, R., Rocca, M. A., Rovaris, M., Bergami, A., Zyroff, J., Scotti, G., & Comi, G. (1999). Method for intracellular magnetic labeling of human mononuclear cells using approved iron contrast agents. Magnetic Resonance Imaging, 17(10), 1521–1523.
Modo, M., Hoehn, M., & Bulte, J. W. (2005). Cellular MR imaging. Molecular Imaging, 4(3), 15353500200505145.
Zhao, M., Beauregard, D. A., Loizou, L., Davletov, B., & Brindle, K. M. (2001). Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nature Medicine, 7(11), 1241.
Frankel, A. D., & Pabo, C. O. (1988). Cellular uptake of the tat protein from human immunodeficiency virus. Cell, 55(6), 1189–1193.
Koch, A. M., Reynolds, F., Kircher, M. F., Merkle, H. P., Weissleder, R., & Josephson, L. (2003). Bioconjugate Chemistry, 14, 1115.
Zhao, M., Kircher, M. F., Josephson, L., & Weissleder, R. (2002). Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconjugate Chemistry, 13(4), 840–844.
Strable, E., Bulte, J. W., Moskowitz, B., Vivekanandan, K., Allen, M., & Douglas, T. (2001). Synthesis and characterization of soluble iron oxide−dendrimer composites. Chemistry of Materials, 13(6), 2201–2209.
Stella, B., Arpicco, S., Peracchia, M. T., Desmaële, D., Hoebeke, J., Renoir, M., D’Angelo, J., Cattel, L., & Couvreur, P. (2000). Design of folic acid-conjugated nanoparticles for drug targeting. Journal of Pharmaceutical Sciences, 89(11), 1452–1464.
Zhang, Y., Kohler, N., & Zhang, M. (2002). Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials, 23(7), 1553–1561.
Perez, J. M., Josephson, L., & Weissleder, R. (2004). Use of magnetic nanoparticles as nanosensors to probe for molecular interactions. Chembiochem, 5(3), 261–264.
Perez, J. M., O’Loughin, T., Simeone, F. J., Weissleder, R., & Josephson, L. (2002). DNA-based magnetic nanoparticle assembly acts as a magnetic relaxation nanoswitch allowing screening of DNA-cleaving agents. Journal of the American Chemical Society, 124(12), 2856–2857.
Perez, J. M., Josephson, L., O’Loughlin, T., Högemann, D., & Weissleder, R. (2002). Magnetic relaxation switches capable of sensing molecular interactions. Nature Biotechnology, 20(8), 816.
Gao, Y. (2005). Biofunctionalization of magnetic nanoparticles. Nanotechnologies for the Life Sciences. Weinheim: Wiley-VCH.
Safarik, I., & Safarikova, M. (2004). Magnetic techniques for the isolation and purification of proteins and peptides. BioMagnetic Research and Technology, 2(1), 7.
Bucak, S., Jones, D. A., Laibinis, P. E., & Hatton, T. A. (2003). Protein separations using colloidal magnetic nanoparticles. Biotechnology Progress, 19(2), 477–484.
Alexiou, C., Schmid, R. J., Jurgons, R., Kremer, M., Wanner, G., Bergemann, C., Huenges, E., Nawroth, T., Arnold, W., & Parak, F. G. (2006). Targeting cancer cells: magnetic nanoparticles as drug carriers. European Biophysics Journal, 35(5), 446–450.
Kandori, K., & Ishikawa, T. (2004). Preparation and microstructural studies on hydrothermally prepared hematite. Journal of Colloid and Interface Science, 272(1), 246–248.
Petcharoen, K., & Sirivat. (2012). Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Materials Science and Engineering B, 177, 421–427.
Valentin, V. M., Svetlana, S. M., Andrew, J. L., Olga, V. S., & Anna, O. D. (2014). Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeumvulgare and Rumexacetosa plants. Langmuir, 30, 5982–5988.
Raveendran, P., Fu, J., & Wallen, S. L. (2003). Completely “green” synthesis and stabilization of metal nanoparticles. Journal of the American Chemical Society, 125, 13940–13941.
Mervat, F. Z., EWael, H. E., & Shabaka, A. A. (2012). Malvaparviflora extract assisted green synthesis of silver nanoparticles. Spectrochimica Acta, Part A, 98, 423–428.
Malik, P. M., Shankar, R., Malik, V., Sharma, N., & Mukherjee, T. K. (2014). Green chemistry based benign routes for nanoparticle synthesis. Journal of Nanoparticles. https://doi.org/10.1155/2014/302429.
Praveen, K. T., Ritesh, C. S., & Santosh, B. (2013). Removal of arsenic(III) from water with clay-supported zerovalent iron nanoparticles synthesized with the help of tea liquor. Industrial and Engineering Chemistry Research, 52, 10052–10058.
Ayman, A. A., Medhat, A. A. G., Mana, F., Mona, B. M., & Abdel-Mohamed, M. S. A. (2013). Phytosynthesis of Au, Ag, and Au–Ag bimetallic nanoparticles using aqueous extract of sago pondweed (Potamogetonpectinatus L). ACS Sustainable Chemistry & Engineering, 1, 1520–1529.
Haung, L., Weng, X., Chen, Z., Megharaj, M., & Naidu, R. (2014). Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 117, 801–804.
Njagi, E. C., Huang, H., Stafford, L., Homer, G., Hugo, M. G., Collins, B. C., et al. (2010). Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir, 27, 264–271.
Smuleac, V., Varma, R., Sikdar, S., & Bhattacharyya, D. (2011). Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. Journal of Membrane Science, 379, 131–137.
Wu, Y., Zeng, S., Wang, F., Megharaj, M., Naidu, R., & Chen, Z. (2015). Heterogeneous Fenton-like oxidation of malachite green by iron-based nanoparticles synthesized by tea extract as a catalyst. Separation and Purification Technology, 154, 161–167.
Shahwan, T., Abu Sirriah, S., Nairat, M., Boyaci, E., Eroglu, A. E., Scott, T. B., & Hallam, K. R. (2011). Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chemical Engineering Journal, 172, 258–266.
Lunge, S., Singh, S., & Sinha, A. (2014). Magnetic iron oxide (Fe3O4) nanoparticles from tea waste for arsenic removal. Journal of Magnetism and Magnetic Materials, 356, 21–31.
Nadagouda, M. N., Castle, A. B., Murdock, R. C., Hussain, S. M., & Varma, R. S. (2010). In vitro biocompatibility of nanoscalezerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chemistry, 12, 114–122.
Alagiri, M., & Abdul Hamid, S. B. (2014). Green synthesis of α-Fe2O3 nanoparticles for photocatalytic application. Journal of Materials Science: Materials in Electronics, 25, 3572–3577.
Harshiny, M., Iswarya, C. N., & Matheswaran, M. (2015). Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaf extract as a reducing agent. Powder Technology, 286, 744–749.
Yizhong, C., Mei, S., & Harold, C. (2003). Antioxidant activity of betalains from plants of the Amaranthaceae. Journal of Agricultural and Food Chemistry, 51, 2288–2294.
Jannathul, M., & Lalitha, P. (2014). Competence of different methods in the biosynthesis of silver nanoparticles. Journal of Chemical and Pharmaceutical Research, 6, 1089–1093.
Ratul, K. D., Nayanmoni, G., Punuri, J. B., Pragya, S., Chandan, M., & Utpal, B. (2012). The synthesis of gold nanoparticles using Amaranthus spinosus leaf extract and study of their optical properties. Advances in Materials Physics and Chemistry, 2, 275–281.
Kumar, B., Kumari, S., Cumbal, L., Debut, A., & Angulo, Y. (2017). Biofabrication of copper oxide nanoparticles using Andean blackberry (RubusglaucusBenth.) fruit and leaf. Journal of Saudi Chemical Society, 21, S475–S480.
Mohanraj, S., Kodhaiyolii, S., Rengasamy, M., & Pugalenthi, V. (2014). Green synthesized iron oxide nanoparticles effect on fermentative hydrogen production by Clostridium acetobutylicum. Applied Biochemistry and Biotechnology, 173, 318–331.
Christensen, L., Vivekanandhan, S., Misra, M., & Mohanty, A. K. (2011). Biosynthesis of silver nanoparticles using Murraya koenigii (curry leaf): an investigation on the effect of broth concentration in reduction mechanism and particle size. Advances Material Letters, 2, 429–434.
Babu, S. A., & Prabu, H. G. (2011). Synthesis of AgNPs using the extract of Calotropisprocera flower at room temperature. Materials Letters, 65, 1675–1677.
Wang, J., & Wan, W. (2008). Effect of Fe2+ concentration on fermentative hydrogen production by mixed cultures. International Journal of Hydrogen Energy, 33, 1215–1220.
Han, H., Cui, M., Wei, L., Yang, H., & Shen, J. (2011). Enhancement effect of hematite nanoparticles on fermentative hydrogen production. Bioresource Technology, 102, 7903–7909.
Prasad, C., Gangadhara, S., & Venkateswarlu, P. (2015). Bio-inspired green synthesis of Fe3O4 magnetic nanoparticles using watermelon rinds and their catalytic activity. Applied Nanoscience, 5, 847–855.
Senthil, M., & Ramesh, C. (2012). Biogenic synthesis of Fe3O4 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Pseudomonas aeruginosa. Digest Journal of Nanomaterials and Biostructures, 7, 1655–1660.
Venkatesh, S., Reddy, Y. S. R., Ramesh, M., Swamy, M. M., Mahadevan, N., & Suresh, B. (2008). Pharmacognostical studies on Dodonaea viscosa. African Journal of Pharmacy and Pharmacology, 2, 083–088.
Kiruba Daniel, S. C. G., Vinothini, G., Subramanian, N., Nehru, K., & Sivakumar, M. M. (2013). Biosynthesis of Cu, ZVI, and Ag nanoparticles using Dodonaea viscosa extract for antibacterial activity against human pathogens. Journal of Nanoparticle Research, 15, 1–10.
Eftekhari, K., Pasha, K. M., Tarigopula, S. P., Sura, M., & Daddam, J. R. (2014). Biosynthesis and characterization of silver and iron nanoparticles from Spinacia oleracea and their antimicrobial studies. International Journal of Plant Animal and Environmental Sciences, 5, 166.
Naseem, T, & Farrukh, M. A. (2015). Antibacterial activity of green synthesis of iron nanoparticles using Lawsonia inermis and Gardenia jasminoides leaves extract. Journal of Chemistry, 1–7. https://doi.org/10.1155/2015/912342.
Makarov, V. V., Love, A. J., Sinitsyna, O. V., Makarova, S. S., Yaminsky, I. V., Taliansky, M. E., et al. (2014). “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae, 6, 35–44.
Kumar, R., Singh, N., & Pandey, N. (2015). Potential of green synthesized zero-valent iron nanoparticles for remediation of lead-contaminated water. Science and Technology, 12, 3943–3950.
Nadagouda, M. N., & Varma, R. S. C. (2007). A greener synthesis of core (Fe, Cu)-shell (Au, Pt, Pd, and Ag) nanocrystals using aqueous vitamin C. Crystal Growth & Design, 7, 2582–2587.
Sun, K., Qiu, J., Liu, J., & Miao, Y. (2009). Preparation and characterization of gold nanoparticles using ascorbic acid as reducing agent in reverse micelles. Journal of Materials Science, 44, 754–758.
Hoag, G. E., Collins, J. B., Holcomb, J. L., Hoag, J. R., Nadagouda, M. N., & Varma, R. S. (2009). Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. Journal of Materials Chemistry, 19, 8671–8677.
Liu, Q., Bei, Y., & Zhou, F. (2009). Removal of lead(II) from aqueous solution with amino-functionalized nanoscale zero-valent iron. Central European Journal of Chemistry, 7, 79–82.
Zhang, X., Lin, S., Lu, X. Q., & Chen, Z. (2010). Removal of Pb(II) from water using synthesized kaolin supported nanoscale zero-valent iron. Chemical Engineering Journal, 163, 243–248.
Li, X., Elliott, D. W., & Zhang, W. (2006). Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Critical Reviews in Solid State and Materials Sciences, 31, 111–122.
O’Carroll, D., Sleep, B., Krol, M., Boparai, H., & Kocur, C. (2013). Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Advances in Water Resources, 51, 104–122.
Venkateswarlu, S., Natesh Kumar, B., Prathima, B., SubbaRao, Y., & Vijaya Jyothi, N. V. (2014) A novel green synthesis of Fe3O4 magnetic nanorodsusing Punica granatum rind extract and its application for removal of Pb(II) from aqueous environment. Arabian Journal of Chemistry. https://doi.org/10.1016/j.arabjc.2014.09.006.
Jang, S. H., Min, B. G., Jeong, Y. G., Lyoo, W. S., & Lee, S. C. (2008). Removal of lead ions in aqueous solution by hydroxyapatite polyurethane composite foams. Journal of Hazardous Materials, 152, 1285–1292.
Wu, S. C., Peng, X. L., Cheng, K. C., Liu, S. L., & Wong, M. H. (2009). Adsorption kinetics of Pb and Cd by two plant growth promoting rhizobacteria. Bioresource Technology, 100, 4559–4563.
Ni, J., Xiong, L., Chen, C., & Chen, Q. (2011). Adsorption of Pb(II) and Cd(II) from aqueous solutions using titanate nanotubes prepared via hydrothermal method. Journal of Hazardous Materials, 189, 741–748.
Shah, F., Kazi, T. G., Afridi, H. I., Khan, S., Kolachi, N. F., Arain, M. B., & Baig, J. A. (2011). The influence of environmental exposure on lead concentrations in scalp hair of children in Pakistan. Ecotoxicology and Environmental Safety, 74, 727–732.
Madadrang, C. J., Kim, H. Y., Gao, G., Wang, N., Zhu, J., Feng, H., et al. (2012). Adsorption behavior of EDTA-graphene oxide for Pb (II) removal. ACS Applied Materials & Interfaces, 4, 1186–1193.
Stafej, A., & Pyrzynska, K. (2007). Adsorption of heavy metal ions with carbon nanotubes. Separation and Purification Technology, 58, 49–52.
Hua, M., Zhang, S., Pan, B., Zhang, W., Lv, L., & Zhang, Q. (2012). Heavy metal removal from water/wastewater by nanosized metal oxides. Journal of Hazardous Materials, 211–212, 317–331.
Hajdu, I., Bodnar, M., Csikos, Z., Wei, S., Daroczi, L., Kovacs, B., et al. (2012). Combined nano-membrane technology for removal of lead ions. Journal of Membrane Science, 409, 44–53.
Suc, N. V., Ho, T. Y., & Ly. (2013). Lead (II) removal from aqueous solution by chitosan flake modified with citric acid via crosslinking with glutaraldehyde. Journal of Chemical Technology and Biotechnology, 88, 1641–1649.
Barakat, M. A., & Schmidt, E. (2010). Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater. Desalination, 256, 90–93.
Uluozlu, O. D., Sari, A., Tuzen, M., & Soylak, M. (2008). Biosorption of Pb(II) and Cr(III) from aqueous solution by lichen (Parmelinatiliaceae) biomass. Bioresource Technology, 99, 2972–2980.
Çam, M., & Hışıl, Y. (2010). Pressurised water extraction of polyphenols from pomegranate peels. Food Chemistry, 123, 878–885.
Wang, T., Jin, X., Chen, Z., Megharaj, M., & Naidu, R. (2014). Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Science of the Total Environment, 466-467, 210–213.
Eneji, A. E., Islam, R., An, P., & Amalu, U. C. (2013). Nitrate retention and physiological adjustment of maize to soil amendment with superabsorbent polymers. Journal of Cleaner Production, 52, 474–480.
Wang, T., Lin, J., Chen, Z., Megharaj, M., & Naidu, R. (2014). Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. Journal of Cleaner Production, 83, 413–419.
Thakur, S., & Karak, N. (2012). Green reduction of graphene oxide by aqueous phytoextracts. Carbon, 50, 5331–5339.
Pattanayak, M., Mohapatra, D., & Nayak, P. L. (2013). Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Syzygium aromaticum (clove). Middle-East Journal of Scientific Research, 18, 623–626.
Balamurughan, M. G., Mohanraj, S., Kodhaiyolii, S., & Pugalenthi, V. (2014). National Conference on Green Engineering and Technologies for Sustainable Future-2014 Ocimum sanctum leaf extract mediated green synthesis of iron oxide nanoparticles: spectroscopic and microscopic studies. National Conference on Green Engineering and Tec 4:201–204.
Lengke, M. F., Fleet, M. E., & Southam, G. (2007). Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir, 23, 2694–2699.
Husseiny, M., El-Aziz, M. A., Badr, Y., & Mahmoud, M. (2007). Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochimica Acta Part A, Molecular and Biomolecular Spectroscopy, 67, 1003–1006.
Xie, J., Lee, J. Y., Wang, D. I., & Ting, Y. P. (2007). Identification of active biomolecules in the high-yield synthesis of single-crystalline gold nanoplates in algal solutions. Small, 3, 672–682.
Chen, J., Lin, Z., & Ma, X. (2003). Evidence of the production of silver nanoparticles via pretreatment of Phoma sp.3.2883 with silver nitrate. Letters in Applied Microbiology, 37, 105–108.
Bhainsa, K. C., & Souza, S. D. (2006). Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigates. Colloids and Surfaces, B: Biointerfaces, 47, 160–164.
Mukherjee, P., Roy, M., Mandal, B., Day, G., Ghatak, J., Tyagi, A., et al. (2008). Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology, 19, 75103–75110.
Mahdavi, M., Namvar, F., Bin Ahmed, M., & Mohamad, R. (2013). Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules, 18, 5954–5964.
Tarafdar, J. C., & Raliya, R. (2013). Rapid, low-cost, and ecofriendly approach for iron nanoparticle synthesis using Aspergillus oryzae TFR9. Journal of Nanoparticles. https://doi.org/10.1155/2013/141274.
Bharde, A., Wani, A., Shouche, Y., Pa, J., Prasad, B. L. V., & Sastry, M. (2005). Bacterial aerobic synthesis of nanocrystalline magnetite. Journal of the American Chemical Society, 127, 9326–9327.
Shenton, W., Douglas, T., Young, M., Stubbs, G., & Mann, S. (1999). Inorganic organic nanotube composites from template mineralization of tobacco mosaic virus. Advanced Materials, 11, 253–256.
Periyathambi, P., Vedakumari, W. S., Bojja, S., Kumar, S. B., & Sastry, T. P. (2014). Green biosynthesis and characterization of fibrin functionalized iron oxide nanoparticles with MRI sensitivity and increased cellular internalization. Materials Chemistry and Physics, 148, 1212–1220.
Widanarto, W., Sahar, M. R., Ghoshal, S. K., Arifin, R., Rohani, M. S., & Hamzah, K. (2013). Effect of natural Fe3O4 nanoparticles on structural and optical properties of Er 3+ doped tellurite glass. Journal of Magnetism and Magnetic Materials, 326, 123–128.
Funding
This work was supported by the Biophysical Scientific Society under the supervision of Biophysics Department, Faculty of Science, Cairo University.
Author information
Authors and Affiliations
Contributions
All the authors researched the existing literatures, wrote the manuscript, designed the manuscript, developed the concept, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Fahmy, H.M., Mohamed, F.M., Marzouq, M.H. et al. Review of Green Methods of Iron Nanoparticles Synthesis and Applications. BioNanoSci. 8, 491–503 (2018). https://doi.org/10.1007/s12668-018-0516-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0516-5