Abstract
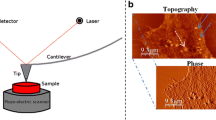
Glioblastoma multiforme (GBM) is a highly invasive (WHO grade IV) brain tumour that has a very poor prognosis for patients with the condition (median survival 14.2 months). Quantitative Imaging (QI)® mode atomic force microscopy (AFM) was used to measure the heights of the leading-edge cell peripheries, the lamellipodia, of two such cell lines (SNB-19 and UP-007), together with those from non-neoplastic astrocyte control cells (CC-2565 and SC-1800) and from a low-grade (WHO grade I) glioma cell line (SEBTA-048). The lamellipodia heights of the glioma cells SNB-19 and UP-007 were 2.45 ± 0.59 and 1.57 ± 0.42 μm, respectively, which were higher than those of the CC-2565 and SC-1800 cells (1.03 ± 0.58 and 0.85 ± 0.40 μm, respectively; p < 0.0001, except between CC-2565 and UP-007, p < 0.001). Lamellipodia height differences between the two glioma cell lines (p < 0.0001) might be attributed to the measured difference in invasive potential between these two cell lines. The equivalent lamellipodia height of the SEBTA-048 cells was 1.16 ± 0.48 μm, the same as that of the astrocytes (p > 0.05) but lower than those of the high-grade gliomas (p < 0.0001 and p < 0.01 for SNB-19 and UP-007, respectively). These measured heights, therefore, may provide new insights for monitoring and controlling cellular invasion in brain tumours.











Similar content being viewed by others
References
Louis, D. N., Ohgaki, H., Wiestler, O. D., Cavenee, W. K., Burger, P. C., Jouvet, A., et al. (2007). The 2007 WHO Classification of tumours of the central nervous system. Acta Neuropathologica, 114, 97–109. doi:10.1007/s00401-007-0243-4.
Hoa, V. K. Y., Reijneveld, J. C., Enting, R. H., Bienfait, H. P., Robe, P., Baumert, B. G., et al. (2014). Changing incidence and improved survival of gliomas. European Journal of Cancer, 50, 2309–2318. doi:10.1016/j.ejca.2014.05.019.
Cross, S. E., Jin, Y. S., Rao, J., Gimzewski, J. K. (2007). Nanomechanical analysis of cells from cancer patients. Nature Nanotechnology, 2, 780–783. doi:10.1038/nnano.2007.388.
Docheva, D., Padula, D., Schieker, M., Clausen-Schaumann, H. (2010). Effect of collagen I and fibronectin on the adhesion, elasticity and cytoskeletal organization of prostate cancer cells. Biochemical and Biophysical Research Communications, 402, 361–366. doi:10.1016/j.bbrc.2010.10.034.
Wang, B., Lancon, P., Bienvenu, C., Vierling, P., Di Giorgio, C., Bossis, G. (2013). A general approach for the microrheology of cancer cells by atomic force microscopy. Micron, 44, 287–297. doi:10.1016/j.micron.2012.07.006.
Vadillo-Rodriguez, V., & Dutcher, J. R. (2009). Dynamic viscoelastic behavior of individual Gram-negative bacterial cells. Soft Matter, 5, 5012–5019. doi:10.1039/b912227c.
Maherally, Z., Smith, J. R., An, Q., Pilkington, G. J. (2012). Receptors for hyaluronic acid and poliovirus: a combinatorial role in glioma invasion? PLoS One, 7, e30691. doi:10.1371/journal.pone.0030691.
Maherally, Z., Smith, J. R., Ghoneim, M. K., Dickson, L., An, Q., Fillmore, H. L., Pilkington, G. J. (2015). Silencing of CD44 in glioma leads to changes in cytoskeletal protein expression and cellular biomechanical deformation properties as measured by AFM nanoindentation. BioNanoScience, 1–11. doi:10.1007/s12668-015-0189-2.
Romet-Lemonne, G., & Jégou, A. (2013). Mechanotransduction down to individual actin filaments. European Journal of Cell Biology, 92, 333–338. doi:10.1016/j.ejcb.2013.10.011.
Ridley, A. J. (2011). Life at the leading edge. Cell, 145, 1012–1022. doi:10.1016/j.cell.2011.06.010.
Svitkina, T. M., & Borisy, G. G. (1999). Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. Journal of Cell Biology, 145, 1009–1026. doi:10.1083/jcb.145.5.1009.
Yamada, H., Ade, T., Li, S.-A., Masuoka, Y., Isoda, M., Watanabe, M., et al. (2015). Dynasore, a dynamic inhibitor, suppresses lamellipodia formation and cancer cell invasion by destabilizing actin filaments. Biochemical and Biophysical Research Communications, 390, 1142–1148. doi:10.1016/j.bbrc.2009.10.105.
Chopinet, L., Formosa, C., Rols, M. P., Duval, R. E., Dague, E. (2013). Imaging living cells surface and quantifying its properties at high resolution using AFM in QI™ mode. Micron, 48, 26–33. doi:10.1016/j.micron.2013.02.003.
An, Q., Fillmore, H. L., Vouri, M., Pilkington, G. J. (2014). Brain tumour cell line authentication, an efficient alternative to capillary electrophoresis by using a microfluidics-based system. Neurooncology, 16, 265–273. doi:10.1093/neuonc/not202.
Pilkington, G. J., Akinwunmi, J., Ognjenovic, N., Rogers, J. P. (1993). Differential binding of anti-CD44 on human gliomas in vitro. Neuroreport, 4, 259–262. doi:10.1097/00001756-199303000-00008.
Hutter, J. L., & Bechhoefer, J. (1993). Calibration of atomic-force microscope tips. Review of Scientific Instruments, 64, 1868–1873. doi:10.1063/1.1143970.
Fillmore, H. L., Chasiotis, I., Cho, S. W., Gillies, G. T. (2003). Atomic force microscopy observations of tumour cell invadopodia: novel cellular nanomorphologies on collagen substrates. Nanotechnology, 14, 73–76. doi:10.1088/0957-4484/14/1/317.
Chasiotis, I., Fillmore, H. L., Gillies, G. T. (2003). Atomic force microscopy measurement of cytostructural elements involved in the nanodynamics of tumour cell invasion. Nanotechnology, 14, 557–561. doi:10.1088/0957-4484/14/5/314.
D’Agostino, D. P., Olson, J. E., Dean, J. B. (2009). Acute hyperoxia increases lipid peroxidation and induces plasma membrane blebbing in human U87 glioblastoma cells. Neuroscience, 159, 1011–1022. doi:10.1016/j.neuroscience.2009.01.062.
Selmeczi, D., Szabo, B., Sajo-Bohus, L., Rozlosnik, N. (2001). Morphological changes in living cell cultures following α-particle irradiation studied by optical and atomic force microscopy. Radiation Measurement, 34, 549–553. doi:10.1016/S1350-4487(01)00226-8.
Bastatas, L., Martinez-Marin, D., Matthews, J., Hashem, J., Lee, Y. J., Sennoune, S., et al. (2012). AFM nano-mechanics and calcium dynamics of prostate cancer cells with distinct metastatic potential. Biochimica et Biophysica Acta, 1820, 1111–1120. doi:10.1016/j.bbagen.2012.02.006.
Zhang, X., Tang, Q., Wu, L., Huang, J., Chen, Y. (2015). AFM visualization of cortical filaments/network under cell-bound membrane vesicles. Biochimica et Biophysica Acta, 1848, 2225–2232. doi:10.1016/j.bbamem.2015.06.025.
Birukova, A. A., Arce, F. T., Moldobaeva, N., Dudek, S. M., Garcia, J. G. N., Lal, R., et al. (2009). Endothelial permeability is controlled by spatially defined cytoskeletal mechanics: atomic force microscopy force mapping of pulmonary endothelial monolayer. Nanomedicine: Nanotechnology, Biology and Medicine, 5, 30–41. doi:10.1016/j.nano.2008.07.002.
Acknowledgments
We thank Drs. Robert Field and Alex Winkle from JPK Instruments, Cambridge, UK, for loan of the NanoWizard 3 AFM instrument, and Brain Tumour Research for support. We also thank Prof. Keyoumars Ashkan and Dr. Stavros Polyzoidis, of the Department of Neurosurgery, King’s College Hospital, London, UK, for providing the brain tumour biopsy sample for establishment as a primary cell line (SEBTA-048).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statement
All cell lines established in-house were conducted in accordance with the National Research Ethics Service (NRES) instructions and under ethics permission 11/SC/0048.
Rights and permissions
About this article
Cite this article
Smith, J.R., Maherally, Z., Higgins, S.C. et al. AFM Observation of Heightened Cell Periphery of High-Grade Glioblastoma Cell Lines. BioNanoSci. 6, 47–53 (2016). https://doi.org/10.1007/s12668-015-0188-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-015-0188-3