Abstract
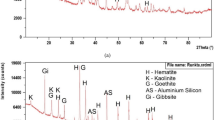
The decomposition temperature is available for pure, synthetic, stoichiometric goethite and a particular type of goethite ore. The decomposition temperature depends on the size of grains, temperature, pressure, heating rate and so on. The decomposition temperature of goethite decides the drying and preheating temperature on induration stand during thermal hardening of the pellets. The induration steps are designed based on the thermal behavior of goethite. The goethite ores, because of their chemically combined moisture content, pose special problems. The generation of fines from iron ore due to internal pressure developed from the evaporation of moisture or phase transformation (28% increases in volume) has a harmful effect on induration of pellets and blast furnace performance. Such behavior of iron-bearing materials is evaluated by thermal degradation index. To avoid/control the cracking/breaking of goethite pellet on induration stand, we should be aware of phase transformation temperature and thermal behavior of goethite. Therefore, we can decide the thermal profile for the pelletization of the goethite ore. Therefore, this study is to find out the decomposition temperature of goethite with respect to the mineralogy of ores and heating rate.








Similar content being viewed by others
References
Biswas A K, Principle of Blast Furnace Iron Making: Theory & Practice, 1st Ed (1981).
Meyer K, Pelletizing of Iron Ores, Springer, Heidelberg (1980).
Jaroslav S R B, and Ruzickova Z, Developments in Mineral Processing 7, “Pelletization of Fines”, Elsevier, Amsterdam (1988).
Ball D F, Dartnell, Grieve J, and Wild A R, Agglomeration of Iron Ores, Elsevier, New York (1973).
Diamandescu L, Mihaila-Tarabasanu D, and Fede M, Mater Lett17 (1993) 309.
Watari F, Delavignette P, Van Landuyt J, and S Amelinckx, J Solid State Chem48 (1983) 49.
Das S K, Das B, Sakthivel R, and Mishra B K, Miner Process Extr Metall Rev31 (2010) 97.
Fey M V, and Dixon J B, Clays Clay Miner29 (1981), 91.
Schulze D G, and Schwertmann U, Clay Miner22 (1987) 83.
Lima-de-faru J, Zeitschrift fiir Kristallographie, Bd. 119 (1963) 176.
Lima-de-fari J, Acta Cryst23 (1967) 733.
Watanabe Y, and Ishii K, Phys Stat Sol. (A)150 (1995) 673.
Francombe M H, and Rooksby H P, Clay Min Bull4 (1959) 1.
Mitov I, Paneva D, and Kunev B, Thermochimica Acta386 (2002) 179.
Walter D, Buxbaum G, and Laqua W J Therm Anal Calorim63 (2001) 733.
F Rafat, Arch Appl Sci Res6 (2014) 75.
Goss C J, Mineral Mag51 (1987) 437.
Coats A V, and Redfern J P, Nature201 (1964) 68.
Liu Q, Yu Y, Torrent J, Roberts A, Yongxin P, and Zhu R, J Geophysics Res111 (2006) 1.
Kyoung-oh J, Nunna V R M, Hapugoda S, Nguyen A V, and Bruckard W J, Minerals Eng60 (2014)14.
Diakonov I, Khodakovsky I, Schott J, and Sergeeva E, Eur J Miner6 (1994) 967.
Acknowledgements
The authors express their sincere gratitude to the Director, CSIR-National Metallurgical Laboratory, to accord permission in publishing the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ammasi, A. Effect of Heating Rate on Decomposition Temperature of Goethite Ore. Trans Indian Inst Met 73, 93–98 (2020). https://doi.org/10.1007/s12666-019-01806-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-019-01806-w