Abstract
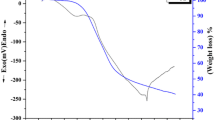
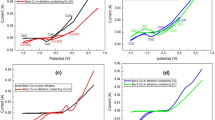
Plasma electrolytic oxidation (PEO) coupled with electrophoretic deposition (EPD) was used to fabricate ZrO2/SiC composite coating on the zirconium metal. The PEO–EPD process was carried out in three different electrolyte systems consisting of 5 g/l sodium aluminate or trisodium orthophosphate or sodium metasilicate with 4 g/l SiC nanoparticles. The X-ray diffraction results indicate monoclinic zirconia is the major phase in phosphate and silicate electrolyte while the coating produced in aluminate electrolyte is composed of tetragonal zirconia. The potentiodynamic polarization studies (PDP) indicate that composite coating produced in phosphate + SiC nanoparticle containing electrolyte exhibit superior resistance to corrosion, which can be attributed to the pore-free morphology of the coating. All the PEO–EPD coatings show exceptionally good adhesion strength (Lc > 40 N). The coating fabricated in phosphate + SiC nanoparticles is found to be the best coating because of its superior resistance to corrosion and reasonably good adhesion strength.













Similar content being viewed by others
References
Raj B, and Mudali U K, Prog Nucl Energ 48 (2006) 283.
Miyake M, Uno M, and Yamanaka S, J Nucl Mater 270 (1999) 233.
Fisher N J, Weckwerth M K, Grandison D A E, and Cotnam B M, Nucl Eng Des 213 (2002) 79.
Sanchez A G, Schreiner W, Duffo G, and Cere S, Appl Surf Sci 257 (2011) 6397.
Leushake U, Krell T, Schulz U, Peters M, Kaysser W A, and Rabin B H, Surf Coat Technol 94–95 (1997) 131.
Brenier R, Mugnier J, and Mirica E, Appl Surf Sci 143 (1999) 85.
Shanmugavelayutham G, Yano S, and Kobayashi A, Vacuum 80 (2006) 1336.
Zhou F Y, Wang B L, Qiu K J, Li H F, Li L, Zheng Y F, and Han Y, Appl Surf Sci 265 (2013) 878.
Klapkiv M D, Povstyana N Y, and Nykyforchyn H M, Mater Sci 42 (2006) 277.
Fatimah S, Kamil M P, Kwon J H, Kaseem M, and Ko Y G, J Alloys Compd 707 (2016) 358.
Hariprasad S, Gowtham S, Arun S, Ashok M, and Rameshbabu N, J Alloys Compd 727 (2017) 698.
Venkateswarlu K, Rameshbabu N, Sreekanth D, Bose A C, Muthupandi V, and Subramanian S, Ceram Int 39 (2013) 801.
Tu W, Cheng Y, Wang X, Zhan T, Han J, and Cheng Y, J Alloys Compd 725 (2017) 199.
Choi J W, Kim G W, Shin K R, Yoo B, and Shin D H, J Alloys Compd 726 (2017) 930.
Sowa M, Woszczak M, Kazek-k A, Dercz G, Korotin D M, Zhidkov I S, and Simka W, Appl Surf Sci 407 (2017) 52.
Parfenov E V, Yerokhin A, Nevyantseva R R, Gorbatkov M V, Liang C J, and Matthews A, Surf Coat Technol 269 (2015) 2.
Cheng Y, Cao J, Peng Z, Wang Q, Matykina E, Skeldon P, and Thompson G E, Electrochim Acta 116 (2014) 453.
Cheng Y, Wu F, Dong J, Wu X, Xue Z, Matykina E, Skeldon P, and Thompson G E, Electrochim Acta 85 (2012) 25.
Cheng Y, and Fan W U, T Nonferr Metal Soc 22 (2012) 1638.
Cheng Y, Wu F, Matykina E, Skeldon P, and Thompson G E Corros Sci 59 (2012) 307.
Zou Z, Xue W, Jia X, Du J, Wang R, and Weng L, Surf Coat Technol 222 (2013) 62.
Sowa M, Dercz G, Suchanek K, and Simka W, Appl Surf Sci 346 (2015) 534.
Arrabal R, Matykina E, Viejo F, Skeldon P, Thompson G E, and Merino M C Appl Surf Sci 254 (2008) 6937.
Matykina E, Arrabal R, Monfort F, Skeldon P, and Thompson G E, Appl Surf Sci 255 (2008) 2830.
Bahramian A, Raeissi K, and Hakimizad A, Appl Surf Sci 351 (2015) 13.
Bai Y, Park I S, Lee S J, Bae T S, Duncan W, Swain M, and Lee M H, Appl Surf Sci 257 (2011) 7010.
Yu L, Cao J, and Cheng Y, Surf Coat Technol 276 (2015) 266.
Nasiri Vatan H, Ebrahimi-kahrizsangi R, and Kasiri-asgarani M, J Alloys Compd 683 (2016) 241.
Lu X, Mohedano M, Blawert C, Matykina E, Arrabal R, and Kainer K U, Surf Coat Technol 307 (2016) 1165.
Aliofkhazraei M, and Rouhaghdam A S, Surf Coat Technol 205 (2011) S57.
Ryong K, Kim Y S, Kim G W, Ko Y G, and Shin D H, Colloids Surf B 131 (2015) 47.
Garvie R C, J Phys Chem 69 (1965) 1238.
Heuer A H, Claussen N, Kriven W M, and Ruhle M, J Am Ceram Soc 65 (1982) 642.
Moros G, Marti M C, Carda J, Tena M A, Escribano P, and Anglada M, J Mater Sci 28 (1993) 5852.
Nagarajan V S, and Rao K J, J Mater Sci 24 (1989) 2140.
Muller E, Oestreich C, Klemm V, Brendler E, Ferkel H, and Riehemann W, Part Part Syst Charact 19 (2002) 169.
Hong J S, De la Torre S D, Miyamoto K, Miyamoto H, and Gao L, Mater Lett 37 (1998) 6.
Yan Y, Han Y, and Huang J, Scr Mater 59 (2008) 203.
K.L. Cheng, The negative charge of nanoparticles, Microchem. J 82 (2006) 119–120.
Cakmat E, Tekin K C, Malayoglu U, and Shrestha S, Surf Coat Technol 204 (2010) 1305.
Nie X, Leyland A, Song H W, Yerokhin A L, Dowey S J, and Matthews A, Surf Coat Technol 116–119 (1999) 1055.
Graeve O A, Shackelford J F, and Doremus R H (Eds.), Ceramic and Glass Materials Structure: Properties and Processing, Springer, New York (2008) p 169.
He Y J,. Winnubst A J A, Schipper D J, Burggraaf A J and Verweij H, Wear 210 (1997) 178.
Chen Y, Nie X, and Northwood D O, Surf Coat Technol 205 (2010) 1774.
Acknowledgements
The authors would like to acknowledge the project grants received from the Department of Science and Technology (DST), New Delhi (Ref. No INT/RUS/RFBR/IDIR/P-5/2016) and Russian Foundation for Basic Research (RFBR), Moscow (Ref. No 16-53-48008) under the DST-RFBR inter-disciplinary scientific cooperation programme to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sukumaran, A., Sampatirao, H., Balasubramanian, R. et al. Formation of ZrO2–SiC Composite Coating on Zirconium by Plasma Electrolytic Oxidation in Different Electrolyte Systems Comprising of SiC Nanoparticles. Trans Indian Inst Met 71, 1699–1713 (2018). https://doi.org/10.1007/s12666-018-1306-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-018-1306-z