Abstract
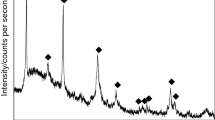
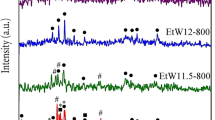
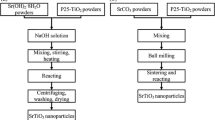
Nano crystalline strontium hexaferrite, SrFe12O19 powders have been synthesized using co-precipitation method. The ferrite precursors were obtained from aqueous mixtures of strontium chloride and ferric chloride by precipitating strontium and iron using 7.5 M sodium hydroxide solution. These precursors were heat treated at 800 and 1200°C in nitrogen atmosphere. The decomposition behaviour and reaction kinetics were investigated by means of DSC/DTG/TG for three heating rates. The various phases associated therein were identified by X-ray diffraction (XRD). From field emission scanning electron microscopy (FESEM) study, the formations of nonporous ultrafine particles have been confirmed. About 5 to 10% of the needle like crystals in the ‘as synthesized, condition were transformed to pyramidal structure and most of the crystals are found to have plate like hexagonal structures with increase in heat treatment temperatures. Activation energies and Avrami index ‘n’ were obtained using Johnson-Mehl-Avrami (JMA) equation. The reaction in the temperature range of 800 to 1200°C, proceeds as, instantaneous nucleation and three dimensional growth with activation energy of 168.33 kJ/mole.
Similar content being viewed by others
References
Palla B J, Shah D O, Casillas P G and Aquino J M, J. Nanopart. Res, 1 (1999) 215.
Costa A C F M, Tortella E, Morelli M R and Kininami R H G A, J. Magn. Mater, 256 (2003) 174.
Qiu J, Gu M and Shen H, J. Magn. Magn. Mater, 295 (2005) 263.
Peng C H, Hwang C C, Wan J, Tsai J S and Chen S Y, Mater. Sci. Eng. B, 117 (2005) 27.
Huang J, Zhuang H and Li W L, Maters. Res. Bull, 38 (2003) 149.
Huang J, Zhuang H and Li W L, J. Magn. Magn. Mater, 256 (2003) 390.
Pankov V V, Pernet M, Germi P and Mollard P, J. Magn. Man. Mater, 120 (1993) 69.
Sato H and Umeda T, J. Mater. Trans, 34 (1993) 76.
Elvin G, Parkin I P P, Bui Q T, Barquin L F, Pankhurst Q A, Komarov A V and Morozov Y G, J. Mater. Sci. Lett., 16 (1997) 1237.
Ataie A, Harris I R and Ponton C B, J. Mater. Sci., 30 (1995) 1429.
Ding J, Miao W F, McCormic P G and Street R, J. Alloys Cmpds, 281 (1998) 32.
Tyagi, S, Agarwala, R C and Agarwala, V, Advanced Materials Research, 67 (2009) 203.
Criado J M and Ortega A, Acta Metal, 35 (1987) 1715.
Doyle C D, J. of appl. Polym. Sci., 6 (1962) 639.
Kumazawa, H, Cho, H M and Sada E, J. Mater. Sci., 28 (1993) 5230.
Ostwald W Z, Phys. Chem, 34 (1900) 495.
Sharma R, Agarwala R C and Agarwala V, Materials Letters, 62 (2008) 2233.
Sharma R, Agarwala R C and Agarwala V, Journal of Nano Research, 2 (2008) 91.
Sharma R, Agarwala, R C and Agarwala V, Advanced Materials Research, 67 (2009) 39.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tyagi, S., Agarwala, R.C. & Agarwala, V. Reaction kinetic studies of strontium hexaferrite nanoparticles synthesized by co-precipitation method. Trans Indian Inst Met 63, 15–19 (2010). https://doi.org/10.1007/s12666-010-0003-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-010-0003-3