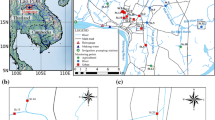
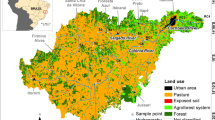
Abstract
Periods with rainfall and rapid snow melting or their simultaneous occurrence are very important for good water quality. Such periods lead to a rapid increase in turbidity as well as the natural organic matter quantity in water. Chlorine as a disinfectant builds the toxic disinfection by-products with the natural organic matter that are harmful to human health. Data monitoring allows the prediction of deterioration of the water quality at the source depending on the climatic parameters. Water supply sources in the catchment area of the Beljevina River, operated by the Bor Water Utility, are an example of well-thought-out monitoring of water quality and quantity, which is a major contributor to the superior public water supply. Given the fundamental role played by turbidity and the lack of studies concerning its magnitude at karst groundwater, the current study has introduced an assessment of this parameter. Consequently, the system of turbidity control is necessary, especially if there is no system to remove turbidity from raw water. Data presented in this paper are a result of the monitoring of the Zlot water supply source used as the supply and distribution system in the City of Bor. The results indicate that the effect of air temperature had a dominant impact on snowmelt, turbidity and water level in the Beljevina River after 2 days. Also, the results showed a significant positive correlation between turbidity and potassium permanganate consumption. Monitoring data of these parameters over a long period will allow the establishment of a simulation model for short-term prediction of turbidity or any other water quality parameters.










Similar content being viewed by others
References
Changa Y, Baia Y, Qua J (2016) Does KMnO4 preoxidation reduce the genotoxicity of disinfection by-products. Chemosphere 163:73–80. https://doi.org/10.1016/j.chemosphere.2016.08.018
Chu W, Gaoa M, Dengb Y, Templeton MR, Yina D (2011) Impacts of drinking water pretreatments on the formation of nitrogenous disinfection by-products. Biores Technol 102:11161–11166. https://doi.org/10.1016/j.biortech.2011.09.109
Daraigan SG, Ahmed S, Wahdain AS, Ahmed S, Bamosa AS, Obid MH (2011) Linear correlation analysis study of drinking water quality data for AlMukallan City, Hadhramout, Yemen. Int J Environ Sci 1:1692–1701
Delpla I, Rodriguez JM (2016) Experimental disinfection by-product formation potential following rainfall events. Water Res 104:340–348. https://doi.org/10.1016/j.watres.2016.08.03
Drinking water—Standard methods for analysis hygienic safety: Federal Institute for Health Protection, NIP Economic review, Beograd, Handbook, (1990).
Egli M, Sartori G, Mirabella A, Giaccai D, Favilli F, Scherner D, Krebs R, Delbos E (2010) The influence of weathering and organic matter on heavy metals lability in silicatic Alpine soils. Sci Total Environ 408:931–946. https://doi.org/10.1016/j.scitotenv.2009.10.005
Golea DM, Upton A, Jarvis P, Moore G, Sutherland S, Parsons SA, Judd SJS (2017) THM and HAA formation from NOM in raw and treated surface waters. Water Res 112:226–235. https://doi.org/10.1016/j.watres.2017.01.051
Hartman A, Goldcheider N, Wagener T, Lange J, Weiler M (2014) Karst water resources in a changing world: review of hydrological modeling approaches. Rev Geophys 52:218–242. https://doi.org/10.1002/2013RG000443
Hurst AM, Edwards MJ, Chipps M, Jefferson B, Parsons SA (2004) The impact of rainstorm events on coagulation and clarifier performance in potable water treatment. Sci Total Environ 321:219–230
Ibrahim MBM, Moursy AS, Radwan EK (2010) Assessment of disinfection by-products (DBPs) formation during chlorination processes. I International conference and exhibition Sustainable water supply and sanitation, The Holding Company for Drinking Water and Wastewater and the Drinking Water and Wastewater and Consumer Protection Agency, Egypt.
Kumar N, Sinha DK (2010) Drinking water quality management through correlation studies among various physico-chemical parameters: a case study. Int J Environ Sci 1(2):253–259
Levchuk I, Jose RM, Sillanpaa M (2018) Removal of natural organic matter (NOM) from water by ion exchange: a review. Chemosphere 192:90–104. https://doi.org/10.1016/j.chemosphere.2017.10.101
Li XF, Mitch WA (2018) Drinking Water disinfection by-products (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ Sci Technol 52:1681–1689. https://doi.org/10.1021/acs.est.7b05440
Maraias SS, Ncube EJ, Msagati TAM, Mamba BB, Nkambule TI (2017) Investigation of natural organic matter (NOM) character and its removal in a chlorinated and chloraminated system at Rand Water, South Africa. Water Supply 17(5):1287–1297. https://doi.org/10.2166/ws.2017.028
Marhaba TF, Van D (2000) The variation of mass and disinfection by-products formation potential of dissolved organic matter fractions along a conventional surface water treatment plant. J Hazard Mater 74:133–147. https://doi.org/10.1016/S0304-3894(99)00190-9
Matilainen A, Lindqvist N, Korhonen S, Tuhkanen T (2002) Removal of NOM in the different stages of the water treatment process. Environ Int 28:457–465. https://doi.org/10.1016/S0160-4120(02)00071-5
Maurya VN, Misra RB, Anderson PK, Vashist S (2016) A case study on water supply access and demand using descriptive statistical methods. Am J Biol Environ Stat 2:7–12
Naceradskaa J, Pivokonskya M, Pivokonskaa L, Baresovaa M, Hendersonc RK, Zamyadic A, Jandad V (2017) The impact of pre-oxidation with potassium permanganate on cyanobacterial organic matter removal by coagulation. Water Res 114:42–49. https://doi.org/10.1016/j.watres.2017.02.029
National drinking water standards (2019) Official Gazette of FRY, nos. 42/98 and 44/99, 28/19.
Nikolaou AD, Golfinopoulos S, Lekkas T, Arhonditis GB (2004) Factor affecting the formation of organic by-products during water chlorination: a bench-scale study. Water Air Soil Pollut 159:357–371. https://doi.org/10.1023/B:WATE.0000049189.61762.61
Nkambule TI, Krause RWM, Mamba BB, Haarhoff J (2009) Characterisation of natural organic matter (NOM) and its removal using cyclodextrin polyurethanes. Water SA 35(2):200. https://doi.org/10.4314/wsa.v35i2.76755
Ostojić Ž, Prodanović D, Marčeta S (2014) Correlation-regression analysis of modeled spatial distribution of residual chlorine in the water supply network. Mater Protect 55:173–180
Petitta M, Bodo B, Caschetto M, Colomban N, Correia V, Cseko A, di Cairano M, Fernandey I, Garcia Alibrand C, Hartai E, Hinsby K, Madarasy T, Mikita V, Garcia PM, Szucs P, van der Keur P (2015) The KINDRA project: a tool for sharing Europe's groundwater research and knowledge. Eur Geol J Eur Fed Geol 40:5–8. https://doi.org/10.7343/as-2018-324
Pešić M, Ristić Vakanjac V, Vakanjac B, Antonijević M, Marković N (2015) Good monitoring as a precondition for high drinking water quality: Case study of Zlot water supply sources (Bor, Serbia). XXIII International Conference Ecological Truth (Ed. Pantović and Marković), University of Belgrade, Technical Faculty Bor, pp 583–589.
Pešić M, Ristić Vakanjac V, Vakanjac B, Jovanov K, Mitrev S (2016) Turbidity simlation for short-term turbidity predictions: case study of the karst spring Surdup (Bor, Serbia). Comptes rendus de l'Acad#emie bulgare des Sciences N 9:1183–1194.
Plewa MJ, Wagner ED (2015) Charting a new path to resolve the adverse health effects of DBPs. In: Karanfil, Tanju, Mitch, Bill, Westerhoff, Paul, Xie, Yuefeng (Eds.), American Chemical Society, pp 3–23, doi: 10.1021/bk-2015-1190.ch001.
Republic Hydrometeorological Service of Serbia—RHMS
Richardson SD (2002) The role of GC-MS and LC-MS in the discovery of drinking water disinfection by-products. J Environ Monit 4:1–9. https://doi.org/10.1039/b105578j
Rodriguez MJ, Serodes JB, Levallois P (2005) Behaviour of trihalomethanes and haloacetic acids in a drinking water distribution system. Water Res 38:4367–4382. https://doi.org/10.1016/j.watres.2004.08.018
Romanov D, Gabrovšek F, Dreybrodt W (2003) Dam sites in soluble rocks: a model of increasing leakage by dissolutional widening of fractures beneath a dam. Eng Geol 70:17–35
Rubiyatno HT, Yanti N, Seng B (2012) The Decrease of organic substance concentration (KMnO4) and turbidity in well (ground) water using biosand filter reactor. J Environ Sci Technol 5:430–440. https://doi.org/10.3923/jest.2012.430.440
Selvam R, Muniraj S, Duraisam T, Muthunarayanan V (2018) Identification of disinfection by-products (DBPs) halo phenols in drinking water. Appl Watersci 8:1–8. https://doi.org/10.1007/s13201-018-0771-1
Senese A, Maugeri M, Vuilleermoz E, Smiraglia C, Diolaiuti G (2014) Using daily air temperature thresholds to evaluate snow melting occurrence and amount on Alpine glaciers by T -index models: the case study of the Forni Glacier (Italy). Cryosphere 8:1921–1933. https://doi.org/10.5194/tc-8-1921-2014
Singer PC (1999) Humic substances as precursors for potentially harmful disinfection by-products. Water Sci Technol 40:25–30. https://doi.org/10.1016/S0273-1223(99)00636-8
Singer PC, Bilyk K (2002) Enhanced coagulation using a magnetic ion exchange resin. Water Res 36(16):4009–4022
Tabari H, Marofi S (2011) Long-term variations of water quality parameters in the Maroon River. Iran Environ Monit Assess 177:273–287. https://doi.org/10.1007/s10661-010-1633-y
Toor R, Mohseni M (2007) UV-H2O2 based AOP and its integration with biological activated carbon treatment for DBP reduction in drinking water. Chemosphere 66(11):2087–2095
Uygner CS, Bekbolet M, Swietlik J (2007) Natural organic matter: definitions and characterization. Advances in Control of Disinfection By-Products in Drinking Water Systems, A. Nikolau, H. Selcuk, L. Rizzo (Derleyenler), Chapter 5.1., pp 253–277, NOVA Science Publishers Inc., NY, USA.
Voza D, Vuković M (2018) The assessment and prediction of temporal variations in surface water quality—a case study. Environ Monit Assess 190:434–450. https://doi.org/10.1007/s10661-018-6814-0
Wang X, Wang J, Zhang Y, Shi Q, Zhang H, Zhang Y, Yang M (2016) Characterization of unknown iodinated disinfection by-products during chlorination/chloramination using ultrahigh resolution mass spectrometry. Sci Total Environ 554:83–88. https://doi.org/10.1016/j.scitotenv.2016.02.157
Xie J, Dongsheng W, John VL, Yanmei Z, Linan X, Christopher WKC (2012) pH modeling for maximum dissolved organic matter removal by enhanced coagulation. J Environ Sci 24(2):276–283
Yingying Z, JeffereyY Y, Yu S, Jill N, Tuqiao Z (2018) The dependence of chlorine decay and DBP formation kinetics on pipe flow properties in drinking water distribution. Water Res 141:32–45. https://doi.org/10.1016/j.watres.2018.04.048
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pešić, M., Milić, S., Nujkić, M. et al. The impact of climatic parameters on the turbidity and natural organic matter content in drinking water in the City of Bor (Eastern Serbia). Environ Earth Sci 79, 267 (2020). https://doi.org/10.1007/s12665-020-09016-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-020-09016-0