Abstract
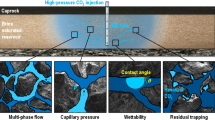
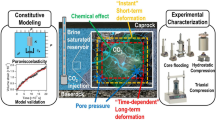
Carbon dioxide (CO2) sequestration in deep saline aquifers is regarded as a potentially useful method of storing CO2 due to their large storage capacity. CO2-trapping mechanisms in such aquifers include solubility trapping, hydrodynamic trapping, structural trapping, and mineral trapping. CO2–water–rock interactions occurring in saline aquifers injected with CO2 are known to play a vital role in these trapping mechanisms. Stress is known to have a significant and positive effect on mineral dissolution, and therefore, pressure solution as a coupled chemo-mechanical behavior could make an important contribution to mineral trapping. Geological storage of CO2 can also be combined with enhanced water recovery (EWR) from deep saline aquifers, a process referred to as CO2–EWR. By exploiting the fluid during CO2–EWR, the pore pressure in the reservoir is altered, which could enhance pressure solution between the mineral grains in the reservoir. In this work, the role played by pore pressure in CO2 mineral trapping from the perspective of pressure solution as a chemo-mechanical coupling process is investigated. To achieve this, seepage–creep tests were performed on sandstone specimens by passing CO2–NaCl solutions through them at different pore pressures. Experimental results show that the lower the pore pressure a specimen is subjected to, the greater the amount of carbon trapped in the sandstone. On the basis of this result, a geometrical model is established for pressure solution in the materials used that quantitatively describes the mechanism responsible for pressure solution. Geometrical model is then used to analyze the effects of the various factors affecting the role played by pressure solution in CO2 mineralization sequestration (mineral type, pore pressure, porosity, and particle size). The results of the analysis are particularly instructive for the evaluation of long-term CO2 storage in terms of pressure solution. As for CO2–EWR, apart from relieving pressure buildup, increasing CO2 injection, regulating CO2 migration, and restricting CO2 leakage, it also enjoys the advantage of enhancing mineral trapping.












Similar content being viewed by others
References
Aagaard P, Oelkers EH, Schott J (2004) Glauconite dissolution kinetics and application to CO2 storage in the subsurface//Geochimica et Cosmochimica Acta. Pergamon-Elsevier Science Ltd 68(11):A143–A143
Ague JJ, Brimhall GH (1989) Geochemical modeling of steady state fluid flow and chemical reaction during supergene enrichment of porphyry copper deposits. Econ Geol 84(3):506–528
Aines RD, Wolery TJ, Bourcier WL, Wolfe T, Hausmann C (2011) Fresh water generation from aquifer-pressured carbon storage: feasibility of treating saline formation waters. Energy Procedia 4:2269–2276
Bachu S (2016) Identification of oil reservoirs suitable for CO2-EOR and CO2 storage (CCUS) using reserves databases, with application to Alberta, Canada. Int J Greenhouse Gas Control 44:152–165
Bachu S, Adams JJ (2003) Sequestration of CO2 in geological media in response to climate change: capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers Manage 44(20):3151–3175
Birkholzer JT, Zhou Q, Tsang CF (2009) Large-scale impact of CO2 storage in deep saline aquifers: a sensitivity study on pressure response in stratified systems. Int J Greenhouse Gas Control 3(2):181–194
Birkholzer JT, Cihan A, Zhou Q (2012) Impact-driven pressure management via targeted brine extraction—conceptual studies of CO2 storage in saline formations. Int J Greenhouse Gas Control 7:168–180
Bond AE, Bruský I, Chittenden N et al (2016) Development of approaches for modelling coupled thermal–hydraulic–mechanical–chemical processes in single granite fracture experiments. Environ Earth Sci 75(19):1313
Buscheck TA, Sun Y, Chen M et al (2012) Active CO2 reservoir management for carbon storage: analysis of operational strategies to relieve pressure buildup and improve injectivity. Int J Greenhouse Gas Control 6:230–245
Buscheck TA, Bielicki JM, White JA et al (2017) Managing geologic CO2 storage with pre-injection brine production in tandem reservoirs. Energy Procedia 114:4757–4764
Carroll SA, Knauss KG (2005) Dependence of labradorite dissolution kinetics on CO2(aq), Al(aq), and temperature. Chem Geol 217:213–225
Coussy O (2004) Poromechanics. Wiley, Chichester
Davidson CL, Dooley JJ, Dahowski RT (2009) Assessing the impacts of future demand for saline groundwater on commercial deployment of CCS in the United States. Energy Procedia 1(1):1949–1956
De Silva GPD, Ranjith PG, Perera MSA (2015) Geochemical aspects of CO2 sequestration in deep saline aquifers: a review. Fuel 155:128–143
Gérard F, Xu T, Brimhall G, Pruess K (1997) Modeling reactive chemical transport problems with the codes EQ3/6 and TRANQUI: Lawrence Berkeley Laboratory Report. California, Berkeley
Ghafoori M, Tabatabaei-Nejad SA, Khodapanah E (2017) Modeling rock-fluid interactions due to CO2 injection into sandstone and carbonate aquifer considering salt precipitation and chemical reactions. J Nat Gas Sci Eng 37:523–538
Gunter WD, Bachu S, Benson S (2004) The role of hydrogeological and geochemical trapping in sedimentary basins for secure geological storage of carbon dioxide. Geol Soc Lond Spec Publ 233(1):129–145
Hangx SJT, Spiers CJ (2009) Reaction of plagioclase feldspars with CO2 under hydrothermal conditions. Chem Geol 265:88–98
Heidug WK (1995) Intergranular solid-fluid phase transformations under stress: the effect of surface forces. J Geophys Res Solid Earth 100(B4):5391–5940
Hunter K, Bielicki JM, Middleton R et al (2017) Integrated CO2 storage and brine extraction. Energy Procedia 114:6331–6336
Jiang Q, Su G, Feng XT, Chen G, Zhang MZ, Liu C (2019) Excavation optimization and stability analysis for large underground caverns under high geostress: a case study of the Chinese Laxiwa project. Rock Mech Rock Eng 52(3):895–915
Kobos PH, Cappelle MA, Krumhansl JL et al (2011) Combining power plant water needs and carbon dioxide storage using saline formations: implications for carbon dioxide and water management policies. Int J Greenhouse Gas Control 5(4):899–910
Labus K, Bujok P, Klempa M et al (2016) Preliminary geochemical modeling of water–rock–gas interactions controlling CO2 storage in the Badenian Aquifer within Czech Part of Vienna Basin. Environ Earth Sci 75(14):1086
Lemieux JM (2011) Review: the potential impact of underground geological storage of carbon dioxide in deep saline aquifers on shallow groundwater resources. Hydrogeol J 19:757–778
Liteanu E, Niemeijer A, Spiers CJ, Peach CJ, Bresser JHP (2012) The effect of CO2 on creep of wet calcite aggregates. J Geophys Res Solid Earth 117:B03211. https://doi.org/10.1029/2011JB008789
Liu J, Sheng J, Polak A et al (2006) A fully-coupled hydrological–mechanical–chemical model for fracture sealing and preferential opening. Int J Rock Mech Min Sci 43(1):23–36
Myrttinen A, Becker V, Nowak M et al (2012) Analyses of pre-injection reservoir data for stable carbon isotope trend predictions in CO2 monitoring: preparing for CO2 injection. Environ Earth Sci 67(2):473–479
Niu Z, Li Q, Wei X et al (2017) Numerical investigation of slippage characteristics of normal and reverse faults under fluid injection and production. Environ Earth Sci 76(14):502
Palandri JL, Rosenbauer RJ, Kharaka YK (2005) Ferric iron in sediments as a novel CO2 mineral trap: CO2–SO2 reaction with hematite. Appl Geochem 20(11):2038–2048
Pietruszczak S, Lydzba D, Shao JF (2006) Modelling of deformation response and chemo-mechanical coupling in chalk. Int J Numer Anal Meth Geomech 30(10):997–1018
Pokrovsky OS, Golubev SV, Schott J et al (2009) Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25–150 °C and 1–55 atm pCO2: new constraints on CO2 sequestration in sedimentary basins. Chem Geol 265(1):20–32
Randolph JB, Saar MO (2011) Coupling carbon dioxide sequestration with geothermal energy capture in naturally permeable, porous geologic formations: implications for CO2 sequestration [J]. Energy Procedia 4:2206–2213
Regnault O, Lagneau V, Catalette H et al (2005) Experimental study of pure mineral phases/supercritical CO2 reactivity. Implications for geological CO2 sequestration. CR Geosci 337:1331–1339
Stephenson LP, Plumley WJ, Palciauskas VV (1992) A model for sandstone compaction by grain interpenetration. J Sediment Res 62(1):11–22
Taron J, Elsworth D (2010) Coupled mechanical and chemical processes in engineered geothermal reservoirs with dynamic permeability. Int J Rock Mech Min Sci 47(8):1339–1348
Yasuhara H, Kinoshita N, Ohfuji H et al (2011) Temporal alteration of fracture permeability in granite under hydrothermal conditions and its interpretation by coupled chemo-mechanical model. Appl Geochem 26(12):2074–2088
Acknowledgements
The authors gratefully acknowledge support by National Natural Science Foundation of China for innovation research groups (Grant no. 51621006) and National Natural Science Foundation of China (General Program Grant nos. 51879261 and 41572296).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, H., Feng, XT., Li, S. et al. Coupled chemo-mechanical behavior of CO2 mineral trapping in the reservoir sandstones during CO2–EWR. Environ Earth Sci 78, 481 (2019). https://doi.org/10.1007/s12665-019-8416-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8416-8