Abstract
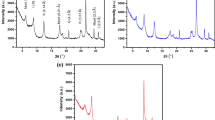
This study describes the removal of Cr3+ ions from aqueous solutions by magnesium oxide (MgO) and montmorillonite nanoparticles (NPs). Optimum values of contact time, adsorbent dosage, temperature, and pH as well as adsorption isotherms, were obtained using concentrations of the metal ions. A scanning electron microscopy-energy dispersive X-ray (SEM–EDX) technique was used to determine adsorbents characteristics. The results showed the maximum uptake values of solutions, by the final point of isotherm experiments were 1033.8 and 3.6 mg/g for MgO nanoparticles (MgO-NPs) and montmorillonite, respectively. The adsorption process followed pseudo-second order kinetic model. Langmuir adsorption isotherm model fitted better to montmorillonite data (R2 = 0.9769), and Freundlich isotherm was in a good agreement with MgO experimental data (R2 = 0.9948). Also, thermodynamic studies were carried out to determine the nature of the process. Thermodynamic results revealed the nature of sorption in MgO-NPs was spontaneous, and in montmorillonite NPs was unspontaneous. Based on the results and by considering the economic aspects of examinations, the MgO-NPs can potentially be used as efficient sorbents for Cr3+ ion removal from aqueous solutions in different conditions.








Similar content being viewed by others
References
Bindu P, Thomas S (2014) Estimation of lattice strain in ZnO nanoparticles: X-ray peakprofile analysis. J Theor Appl Phys 8:123–134
Brigatti MF, Galan E, Theng BKG (2013) Structure and mineralogy of clay minerals. In: Bergaya F, Lagaly G (eds) Handbook of clay science, part A, 2nd edn. Elsevier, Amsterdam, pp 21–82
Cai Y, Li C, Wu D, Wang W, Tan F, Wang X, Wong PK, Qiao X (2017) Highly active MgO nanoparticles for simultaneous bacterial inactivation and heavy metal removal from aqueous solution. J Chem Eng 312:158–166
Demirbaş A (2005) Adsorption of Cr(III) and Cr(VI) ions from aqueous solutions on to modified lignin. Energy Sour 27:1449–1455. https://doi.org/10.1080/009083190523352
Elabbas S, Mandi L, Berrekhis F, Pons MN, Leclerc JP, Ouazzani N (2015) Removal of Cr(III) from chrome tanning wastewater by adsorption using two natural carbonaceous materials: eggshell and powdered marble. J Environ Manag 166:589–595
El-Sherif IY, Tolani S, Ofosu K, Mohamed OA, Wanekaya AK (2013) Polymericnanofibers for the removal of Cr(III) from tannery waste water. J Environ Manag 129:410–413
Essington ME (2015) Soil and water chemistry: an integrative approach. CRC Press, Boca Raton
Fabbricino M, Naviglio B, Tortora G, d’Antonio L (2013) An environmental friendly cycle for Cr(III) removal and recovery from tannery wastewater. J Environ Manag 117:1–6
Feng J, Zou L, Wang Y, Li B, He X, Fan Z, Ren Y, Lv Y, Zhang M, Chen D (2015) Synthesis of high surface area, mesoporous MgO nanosheets with excellent adsorption capability for Ni(II) via a distillation treating. J Colloid Interface Sci 438:259–267
Fonseca-Correa R, Giraldo L, Moreno-Piraján JC (2013) Trivalent chromium removal from aqueous solution with physically and chemically modified corncob waste. J Anal Appl Pyrol 101:132–141
Gupta VK, Ali I, Saleh TA, Siddiqui MN, Agarwal S (2013) Chromium removal from water by activated carbon developed from waste rubber tires. Environ Sci Pollut Res 20:1261–1268
Helmy AK, Ferreiro EA, De Bussetti SG (1994) Cation exchange capacity and condition of zero charge of hydroxy-A1 montmorillonite. Clays Clay Miner 42:444–450
Hooda P (2010) Trace elements in soils. Wiley, Hoboken
Li J, Bai J, Huang K, Zhou B, Wang Y, Hu X (2014) Removal of trivalent chromium in the complex state of trivalent chromium passivation wastewater. J Chem Eng 236:59–65
Liu C, Xie X, Zhao W, Liu N, Maraccini PA, Sassoubre LM, Boehm AB, Cui Y (2013) Conducting nanosponge electroporation for affordable and high-efficiency disinfection of bacteria and viruses in water. Nano Lett 13:4288–4293
Madrakian T, Afkhami A, Zadpour B, Ahmadi M (2015) New synthetic mercaptoethylamino homopolymer-modified maghemite nanoparticles for effective removal of some heavy metal ions from aqueous solution. J Ind Eng Chem 21:1160–1166
Mahdavi S, Amini N (2016) The role of bare and modified nano nickel oxide as efficient adsorbents for the removal of Cd2+, Cu2+, and Ni2+ from aqueous solution. Environ Earth Sci 75:1468. https://doi.org/10.1007/s12665-016-6270-5
Mahdavi S, Jalali M, Afkhami A (2012) Removal of heavy metals from aqueous solutions using Fe3O4, ZnO, and CuO nanoparticles. In: Diallo MS, Fromer NA, Jhon MS (eds) Nanotechnology for sustainable development. Springer, Berlin, pp 171–188. https://doi.org/10.1007/978-3-319-05041-6_14
Mahdavi S, Jalali M, Afkhami A (2013) Heavy metals removal from aqueous solutions using TiO2, MgO, and Al2O3 nanoparticles. Chem Eng Commun 200:448–470
Mahdavi S, Jalali M, Afkhami A (2015) Heavy metals removal from aqueous solutions by Al2O3 nanoparticles modified with natural and chemical modifiers. Clean Tech Environ Policy 17:85–102
Mahdavi S, Molodi P, Zarabi M (2018) Functionalized MgO, CeO2 and ZnO nanoparticles with humic acid for the study of nitrate adsorption efficiency from water. Res Chem Intermed. https://doi.org/10.1007/s11164-018-3408-y
Mahmoud HR, El-Molla SA, Saif M (2013) Improvement of physicochemical properties of Fe2O3/MgO nanomaterials by hydrothermal treatment for dye removal from industrial wastewater. Powder Technol 249:225–233
Marofi S, Zare MA, Krimi Y (2017) Methylene blue removal from aqueous media using tassel adsorbent. Water Sci Technol. https://doi.org/10.2166/wst.2017.347
Mazloomi F, Jalali M (2016) Ammonium removal from aqueous solutions by natural Iranian zeolite in the presence of organic acids, cations and anions. J Environ Chem Eng 4:1664–1673
Mende M, Schwarz D, Steinbach C, Blodt R, Schwarz S (2016) Simultaneous adsorption of heavy metal ions and anions from aqueous solutions on chitosan—investigated by spectrophotometry and SEM-EDX analysis. Colloids Surf A Physichem Eng Asp 510:275–282
Sparks DL (2003) Environmental soil chemistry. Elsevier, Amsterdam
Tahir SS, Naseem R (2007) Removal of Cr(III) from tannery wastewater by adsorption onto bentonite clay. Sep Purif Technol 53:312–321
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. J Chem Eng 308:438–462
Valle JP, Gonzalez B, Schulz J, Salinas D, Romero U, Gonzalez DF, Valdes C, Cantu JM, Eubanks TM, Parsons JG (2017) Sorption of Cr(III) and Cr(VI) to K2Mn4O9 nanomaterial a study of the effect of pH, time, temperature and interferences. Microchem J 133:614–621
Venkatesha TG, Arthoba Nayaka Y, Chethana BK (2013) Adsorption of Ponceau S from aqueous solution by MgO nanoparticles. Appl Surf Sci 276:620–627
Vilvanathan S, Shanthakumar S (2014) Removal of Ni(II) and Co(II) ions from aqueous solution using teak (Tectonagrandis) leaves powder: adsorption kinetics, equilibrium and thermodynamics study. Desalin Water Treat. https://doi.org/10.1080/19443994.2014.989913
Wang S, Choi JW (2013) Simulating fate and transport of chromium in saturated sediments. Appl Math Model 37:102–111
Wu P, Zhang Q, Dai Y, Zhu N, Dang Z, Li P, Wu J, Wang X (2011) Adsorption of Cu(II), Cd(II) and Cr(III) ions from aqueous solutions on humic acid modified Ca-montmorillonite. Geoderma 164:215–219
Xiong C, Wang W, Tan F, Luo F, Chen J, Qiao X (2015) Investigation on the efficiency and mechanism of Cd(II) and Pb(II) removal from aqueous solutions using MgO nanoparticles. J Hazard Mater 299:664–674
Yang ZH, Wang B, Chai LY, Wang YY, Wang HY, Su CQ (2009) Removal of Cr(III) and Cr(VI) from aqueous solution by adsorption on sugarcane pulp residue. J Cent South Univ Technol 16:0101–0107
Acknowledgements
The authors would like to express their gratitude to Bu-Ali Sina University for the support of their work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seif, S., Marofi, S. & Mahdavi, S. Removal of Cr3+ ion from aqueous solutions using MgO and montmorillonite nanoparticles. Environ Earth Sci 78, 377 (2019). https://doi.org/10.1007/s12665-019-8380-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8380-3