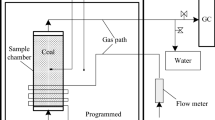
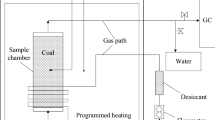
Abstract
Liquid carbon dioxide has an excellent ability of endothermic cooling and inhibition on coal fire, which was an effective coal spontaneous combustion prevention technology. To analyze the oxidation characteristics and variation of apparent activation energies, a carboniferous–permian coal sample was investigated in O2/N2 and O2/CO2 atmospheres by the coal spontaneous combustion oxidation and the Fourier transform infrared spectroscopy experiments. The results indicated that with temperature, carbon monoxide (CO) concentration and oxygen (O2) consumption rate increased. While O2 concentration decreased, CO concentration and oxygen consumption rate reduced. At the same O2 concentration, the oxygen consumption rate and CO concentration on the O2/CO2 atmospheres were less than on the O2/N2 atmospheres. Therefore, O2 concentration reduced, or added CO2 significantly inhibited coal oxidation. As the temperature elevated, the apparent activation energy gradually increased. Furthermore, the apparent activation energy increased when the oxygen concentration reduced in the physical–chemical adsorption stage and the slow oxidation stage. In the rapid oxidation stage, the apparent activation energy lessened with increase in oxygen concentration. Through correlation analysis, the key functional groups in the physical–chemical adsorption stage were hydroxyl, C–O, –COO–, and aliphatic hydrocarbons. During the slow oxidation, the key functional groups were –COO– and aliphatic hydrocarbons. The key functional groups in the rapid oxidation stage were hydroxyl and C–O.






Similar content being viewed by others
References
Arisoy A, Beamish B (2015) Reaction kinetics of coal oxidation at low temperatures. Fuel 159:412–417
Baris K, Kizgut S, Didari V (2012) Low-temperature oxidation of some turkish coals. Fuel 93:423–432
Beamish BB, Barakat MA, George JDS (2001) Spontaneous-combustion propensity of new zealand coals under adiabatic conditions. Int J Coal Geol 45:217–224
Bo L, Gang C, Hui Z, Sheng C (2014) Development of non-isothermal TGA–DSC for kinetics analysis of low temperature coal oxidation prior to ignition. Fuel 118:385–391
Carras JN, Young BC (1994) Self-heating of coal and related materials: models, application and test methods. Prog Energ Combust 20:1–15
Colaizzi GJ (2004) Prevention, control and/or extinguishment of coal seam fires using cellular grout. Int J Coal Geol 59:75–81
Dalverny LE, Chaiken RE (1991) Mine fire diagnostics and implementation of water injection with fume exhaustion at Renton. U.S. Department of the Interior Bureau of Mines Ri, Pittsburgh,, PA
Deng J, Xu J, Li L, Zhang X, Wen H (1999) Investigation on the relation of the rate of oxygen consumption and the size of coal sample. J Xi’an Jiaotong Univ 33:106–107 (in Chinese)
Deng J, Wang K, Zhang Y, Yang H (2014) Study on the kinetics and reactivity at the ignition temperature of Jurassic coal in north Shaanxi. J Therm Anal Calorim 118:417–423
Deng J, Xiao Y, Li QW, Lu J, Wen H (2015a) Experimental studies of spontaneous combustion and anaerobic cooling of coal. Fuel 157:261–269
Deng J, Xiao Y, Lu J, Wen H, Jin Y (2015b) Application of composite fly ash gel to extinguish outcrop coal fires in China. Nat Hazards 79:881–898
Deng J, Ren LF, Ma L, Lei CK, Wei GM, Wang WF (2018) Effect of oxygen concentration on low-temperature exothermic oxidation of pulverized coal. Thermochim Acta 653:102–111
Dou G, Wang D, Zhong X, Qin B (2014) Effectiveness of catechin and poly(ethylene glycol) at inhibiting the spontaneous combustion of coal. Fuel Process Technol 120:123–127
Fierro V, Miranda JL, Romero C, Andres JM, Arriaga A, Schmal D, Visser GH (1999) Prevention of spontaneous combustion in coal stockpiles: experimental results in coal storage yard. Fuel Process Technol 59:23–34
Given PH, Marzec A, Barton WA, Lynch LJ, Gerstein BC (1986) The concept of a mobile or molecular phase within the macromolecular network of coals: a debate. Fuel 65:155–163
Green U, Aizenshtat Z, Metzger L, Cohen H (2012) Field and laboratory simulation study of hot spots in stockpiled bituminous coal. Energy Fuels 26:7230–7235
Herbig C, Jess A (2002) Determination of reactivity and ignition behaviour of solid fuels based on combustion experiments under static and continuous flow conditions. Fuel 81:2387–2395
Huang X, Wei C, Sun W, Jiang C, Feng Y, Xue Y (2014) Investigation of oxygen-containing group promotion effect on CO2–coal interaction by density functional theory. Appl Surf Sci 299:162–169
Khatami R, Stivers C, Levendis YA (2012) Ignition characteristics of single coal particles from three different ranks in O2/N2, and O2/CO2, atmospheres. Combust Flame 159:3554–3568
Kim J, Lee Y, Ryu C, Park HY, Lim H (2015) Low-temperature reactivity of coals for evaluation of spontaneous combustion propensity. Korean J Chem Eng 32:1297–1304
Lee HH, Kim HJ, Yao S, Keffer D, Lee CH (2013) Competitive adsorption of CO2 /CH4, mixture on dry and wet coal from subcritical to supercritical conditions. Chem Eng J 230:93–101
Liu Y, Fu PF, Zhang B, Zheng C (2016) Study on the surface active reactivity of coal char conversion in O2/CO2 and O2/N2 atmospheres. Fuel 181:1244–1256
Nugroho YS, McIntosh AC, Gibbs BM (2000) Low-temperature oxidation of single and blended coals. Fuel 79:1951–1961
Petroleum B (2017) Statistical review of world energy. https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html. Accessed 31 July 2018
Qi G, Wang D, Zheng K, Xu J, Qi X, Zhong X (2015) Kinetics characteristics of coal low-temperature oxidation in oxygen-depleted air. J Loss Prev Process Ind 35:224–231
Qin B, Lu Y, Li Y, Wang D (2014) Aqueous three-phase foam supported by fly ash for coal spontaneous combustion prevention and control. Adv Powder Technol 25:1527–1533
Saini V, Gupta RP, Arora MK (2016) Environmental impact studies in coalfields in India: a case study from Jharia coal-field. Renew Sust Energ Rev 53:1222–1239
Shao ZL, Wang DM, Wang YM, Zhong XX, Tang XF, Hu XM (2015) Controlling coal fires using the three-phase foam and water mist techniques in the Anjialing Open Pit Mine, China. Nat Hazards 75:1833–1852
Shu YB, Li WJ, Li ZX (2012) The technology of liquid CO2 used for fire prevention and the related device. Adv Mater Res 347–353:1642–1646
Singh AK, Singh RVK, Singh MP, Chandra H, Shukla NK (2007) Mine fire gas indices and their application to Indian underground coal mine fires. Int J Coal Geol 69:192–204
Su HT, Song XL, Shi BB (2015) Correlation between oxygen concentration and dynamic parameters of low temperature coal oxidation. Coal Eng 11:120–123
Su H, Zhou F, Li J, Qi H (2017) Effects of oxygen supply on low-temperature oxidation of coal: a case study of Jurassic coal in Yima, China. Fuel 202:446–454
Wang H, Dlugogorski BZ, Kennedy EM (2004) Coal oxidation at low temperatures: oxygen consumption, oxidation products, reaction mechanism and kinetic modeling. Cheminform 35:487–513
Wang DM, Xin HH, Qi XY, Dou GL, Qi GS, Ma LY (2016) Reaction pathway of coal oxidation at low temperatures: a model of cyclic chain reactions and kinetic characteristics. Combust Flame 163:447–460
Xie J, Xue S, Cheng W, Wang G (2011) Early detection of spontaneous combustion of coal in underground coal mines with development of an ethylene enriching system. Int J Coal Geol 85:123–127
Xu Y, Wang L, Chu T, Liang D (2014) Suspension mechanism and application of sand-suspended slurry for coalmine fire prevention. Int J Mining Sci Technol 24:649–656
Zhang Y, Wang J, Wu J, Xue S, Li Z, Chang L (2015) Modes and kinetics of CO2, and CO production from low-temperature oxidation of coal. Int J Coal Geol 140:1–8
Zhang L, Shi B, Qin B, Wu Q, Dao V (2017) Characteristics of foamed gel for coal spontaneous combustion prevention and control. Combust Sci Technol 189:980–990
Zhang T, Bao R, Li S, Zhang C, Zhang L (2018) Expansion properties and creep tests for a new type of solidified expansive sealing material for gas drainage boreholes in underground mines. Environ Earth Sci 77:468
Zhong XX, Wang DM, Yin XD (2010) Test method of critical temperature of coal spontaneous combustion based on the temperature programmed experiment. J China Coal Soc 35:128–131 (in Chinese)
Zhou FB, Shao H, Li JH, Zhang X, Shi BB (2010) Experimental research on combustion product formation during coal spontaneous combustion under reduced oxygen concentrations. J China Univ Min Technol 39:808–812 (in Chinese)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 5157-4139, 5160-4215, and 5180-4247).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, J., Ren, LF., Ma, L. et al. Low-temperature oxidation and reactivity of coal in O2/N2 and O2/CO2 atmospheres, a case of carboniferous–permian coal in Shaanxi, China. Environ Earth Sci 78, 234 (2019). https://doi.org/10.1007/s12665-019-8244-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8244-x