Abstract
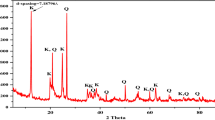
Removal of dyes by low-cost adsorbents is an effective method in wastewater treatment. Iranian natural clays were determined to be effective adsorbents for removal of a basic dye (methylene blue) from aqueous solutions in batch processes. Characterizations of the clays were carried out by X-ray diffraction, Brunauer–Emmett–Teller surface area analysis and field-emission scanning electron microscopy. Effects of the operational parameters such as adsorbent dosage, initial dye concentration, solution pH and temperature were investigated on the adsorption performance. Adsorption isotherms like Langmuir, Freundlich and Temkin were used to analyze the adsorption equilibrium data and Langmuir isotherm was the best fit. Adsorption kinetics was investigated by pseudo-first-order, pseudo-second-order and intraparticle diffusion models and the results showed that the adsorption system conforms well to the pseudo-second-order model. The thermodynamic parameters of adsorption (ΔS°, ΔH° and ΔG°) were obtained and showed that the adsorption processes were exothermic.










Similar content being viewed by others
References
Ahmaruzzaman M, Gayatri SL (2010) Batch adsorption of 4-nitrophenol by acid activated jute stick char: equilibrium, kinetic and thermodynamic studies. Chem Eng J 158:173–180
Ai L, Zhou Y, Jiang J (2011) Removal of methylene blue from aqueous solution by montmorillonite/CoFe2O4 composite with magnetic separation performance. Desalination 266:72–77
Akçay M (2004) Characterization and determination of the thermodynamic and kinetic properties of p-CP adsorption onto organophilic bentonite from aqueous solution. J Colloid Interface Sci 280:299–304
Aksu Z, Kabasakal E (2004) Batch adsorption of 2,4-dichlorophenoxy-acetic acid (2,4-D) from aqueous solution by granular activated carbon. Sep Purif Technol 35:223–240
Almeida C, Debacher N, Downs A, Cottet L, Mello C (2009) Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J Colloïd Interface Sci 332:46–53
Auta M, Hameed B (2013) Acid modified local clay beads as effective low-cost adsorbent for dynamic adsorption of methylene blue. J Ind Eng Chem 19:1153–1161
Banat F, Al-Asheh S, Al-Makhadmeh L (2003) Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem 39:193–202
Barka N, Assabbane A, Nounah A, Laanab L, Ichou YA (2009) Removal of textile dyes from aqueous solutions by natural phosphate as a new adsorbent. Desalination 235:264–275
Barnes G, Gentle I (2011) Interfacial science: an introduction. Oxford University Press, Oxford
Bilgiç C (2005) Investigation of the factors affecting organic cation adsorption on some silicate minerals. J Colloid Interface Sci 281:33–38
Bulut Y, Aydın H (2006) A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194:259–267
Chieng HI, Lim LBL, Priyantha N (2015) Enhancing adsorption capacity of toxic malachite green dye through chemically modified breadnut peel: equilibrium, thermodynamics, kinetics and regeneration studies. Environ Technol 36:86–97
Crini G, Peindy HN, Gimbert F, Robert C (2007) Removal of CI Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol 53:97–110
De Stefanis A, Tomlinson A (2006) Towards designing pillared clays for catalysis. Catal Today 114:126–141
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J Hazard Mater 167:1–9
Deng H-M, Chen Y-H, Wu H-H, Liu T, Wang Y-L, Wu G-Y, Ye H-P (2016) Adsorption of Tl(I) on Na–montmorillonite and kaolinite from aqueous solutions. Environ Earth Sci 75:752
Duman O, Ayranci E (2010) Adsorptive removal of cationic surfactants from aqueous solutions onto high-area activated carbon cloth monitored by in situ UV spectroscopy. J Hazard Mater 174:359–367
Elmoubarki R, Mahjoubi F, Tounsadi H, Moustadraf J, Abdennouri M, Zouhri A, El Albani A, Barka N (2015) Adsorption of textile dyes on raw and decanted Moroccan clays: kinetics, equilibrium and thermodynamics. Water Res Ind 9:16–29
El-Sharkawy E, Soliman AY, Al-Amer KM (2007) Comparative study for the removal of methylene blue via adsorption and photocatalytic degradation. J Colloid Interface Sci 310:498–508
Errais E, Duplay J, Darragi F, M’Rabet I, Aubert A, Huber F, Morvan G (2011) Efficient anionic dye adsorption on natural untreated clay: kinetic study and thermodynamic parameters. Desalination 275:74–81
Ghosh D, Bhattacharyya KG (2002) Adsorption of methylene blue on kaolinite. Appl Clay Sci 20:295–300
Guiza S, Bagane M, Al-Soudani A, Amore HB (2004) Adsorption of basic dyes onto natural clay. Adsorpt Sci Technol 22:245–255
Gupta V (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manag 90:2313–2342
Gürses A, Doğar Ç, Yalçın M, Açıkyıldız M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater 131:217–228
Hameed B, Ahmad A, Aziz N (2009) Adsorption of reactive dye on palm-oil industry waste: equilibrium, kinetic and thermodynamic studies. Desalination 247:551–560
Han R, Zhang J, Han P, Wang Y, Zhao Z, Tang M (2009) Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem Eng J 145:496–504
Ho Y-S (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Ho Y-S, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Jain A, Gupta V, Bhatnagar A (2003) Utilization of industrial waste products as adsorbents for the removal of dyes. J Hazard Mater 101:31–42
Kabra AN, Khandare RV, Govindwar SP (2013) Development of a bioreactor for remediation of textile effluent and dye mixture: a plant–bacterial synergistic strategy. Water Res 47:1035–1048
Kim M-J (2014) A study on the adsorption characteristics of cadmium and zinc onto acidic and alkaline soils. Environ Earth Sci 72:3981–3990
Kooh MRR, Lim LBL, Dahri MK, Lim LH, Bandara JS (2015) Azolla pinnata: an efficient low cost material for removal of methyl violet 2B by using adsorption method. Waste Biomass Valoriz 6:547–559
Kooh MRR, Dahri MK, Lim LBL, Lim LH, Malik OA (2016) Batch adsorption studies of the removal of methyl violet 2B by soya bean waste: isotherm, kinetics and artificial neural network modelling. Environ Earth Sci 75:783
Laidler K, Meiser J (1999) Physical chemistry. Houghton Mifflin, New York, p 852
Langmuir I (1916) The constitutionand fundumental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295
Lim LBL, Priyantha N, Tennakoon D, Dahri MK (2012) Biosorption of cadmium (II) and copper (II) ions from aqueous solution by core of Artocarpus odoratissimus. Environ Sci Pollut Res 19:3250–3256
Lim LBL, Priyantha N, Tennakoon D, Chieng H, Bandara C (2013) Sorption characteristics of peat of Brunei Darussalam I: characterization of peat and adsorption equilibrium studies of methylene blue—peat interactions. Ceylon J Sci (Phys Sci) 17:41–51
Lim LBL, Priyantha N, Chan CM, Matassan D, Chieng HI, Kooh M (2014) Adsorption behavior of methyl violet 2B using duckweed: equilibrium and kinetics studies. Arab J Sci Eng 39:6757–6765
Lim LBL, Priyantha N, Hei Ing C, Khairud Dahri M, Tennakoon D, Zehra T, Suklueng M (2015) Artocarpus odoratissimus skin as a potential low-cost biosorbent for the removal of methylene blue and methyl violet 2B. Desalin Water Treat 53:964–975
Liu P, Zhang L (2007) Adsorption of dyes from aqueous solutions or suspensions with clay nano-adsorbents. Sep Purif Technol 58:32–39
Liu Y, Zheng Y, Wang A (2010) Enhanced adsorption of Methylene Blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J Environ Sci 22:486–493
Mohan D, Singh KP, Singh G, Kumar K (2002) Removal of dyes from wastewater using flyash, a low-cost adsorbent. Ind Eng Chem Res 41:3688–3695
Mondal B, Srivastava VC, Kushwaha JP, Bhatnagar R, Singh S, Mall ID (2013) Parametric and multiple response optimization for the electrochemical treatment of textile printing dye-bath effluent. Sep Purif Technol 109:135–143
Neumann MG, Gessner F, Schmitt CC, Sartori R (2002) Influence of the layer charge and clay particle size on the interactions between the cationic dye methylene blue and clays in an aqueous suspension. J Colloid Interface Sci 255:254–259
Nikagolla C, Chandrajith R, Weerasooriya R, Dissanayake C (2013) Adsorption kinetics of chromium (III) removal from aqueous solutions using natural red earth. Environ Earth Sci 68:641–645
Priyantha N, Lim L, Dahri M (2015) Dragon fruit skin as a potential biosorbent for the removal of methylene blue dye from aqueous solution. Int Food Res J 22(5):2141–2148
Purkait M, Gusain D, DasGupta S, De S (2005) Adsorption behavior of chrysoidine dye on activated charcoal and its regeneration characteristics by using different surfactants. Sep Sci Technol 39:2419–2440
Qiu H, Lv L, B-c Pan, Q-j Zhang, W-m Zhang, Q-x Zhang (2009) Critical review in adsorption kinetic models. J Zhejiang Univ Sci A 10:716–724
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Rida K, Bouraoui S, Hadnine S (2013) Adsorption of methylene blue from aqueous solution by kaolin and zeolite. Appl Clay Sci 83:99–105
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Biores Technol 77:247–255
Shichi T, Takagi K (2000) Clay minerals as photochemical reaction fields. J Photochem Photobiol C 1:113–130
Temkin M, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:217–222
Tsai W, Lai C, Hsien K (2003) Effect of particle size of activated clay on the adsorption of paraquat from aqueous solution. J Colloid Interface Sci 263:29–34
Tsai W, Chang C, Ing C, Chang C (2004) Adsorption of acid dyes from aqueous solution on activated bleaching earth. J Colloid Interface Sci 275:72–78
Ugurlu M, Gurses A, Yalcin M, Dogar C (2005) Removal of phenolic and lignin compounds from bleached kraft mill effluent by fly ash and sepiolite. Adsorption 11:87–97
Vergili I, Kaya Y, Sen U, Gönder ZB, Aydiner C (2012) Techno-economic analysis of textile dye bath wastewater treatment by integrated membrane processes under the zero liquid discharge approach. Resour Conserv Recycl 58:25–35
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manage 93:154–168
Wang L, Zhang J, Zhao R, Li C, Li Y, Zhang C (2010) Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: equilibrium, kinetic and thermodynamic studies. Desalination 254:68–74
Yagub MT, Sen TK, Ang M (2014) Removal of cationic dye methylene blue (MB) from aqueous solution by ground raw and base modified pine cone powder. Environ Earth Sci 71:1507–1519
Yu X, Wei C, Wu H (2015) Effect of molecular structure on the adsorption behavior of cationic dyes onto natural vermiculite. Sep Purif Technol 156:489–495
Yue D, Jing Y, Ma J, Xia C, Yin X, Jia Y (2011) Removal of Neutral Red from aqueous solution by using modified hectorite. Desalination 267:9–15
Yuh-Shan H (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59:171–177
Zehra T, Priyantha N, Lim LBL (2016) Removal of crystal violet dye from aqueous solution using yeast-treated peat as adsorbent: thermodynamics, kinetics, and equilibrium studies. Environ Earth Sci 75:357
Zeng Q, Yu A, Lu G, Paul D (2005) Clay-based polymer nanocomposites: research and commercial development. J Nanosci Nanotechnol 5:1574–1592
Zollinger H (2002) Synthesis, properties and applications of organic dyes and pigments. Colour chemistry. Wiley, New York
Acknowledgements
We thank the University of Tabriz (Iran) for the support provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghdasinia, H., Asiabi, H.R. Adsorption of a cationic dye (methylene blue) by Iranian natural clays from aqueous solutions: equilibrium, kinetic and thermodynamic study. Environ Earth Sci 77, 218 (2018). https://doi.org/10.1007/s12665-018-7342-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7342-5