Abstract
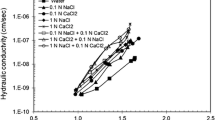
The influence of alkaline aqueous solutions on the properties of bentonite was investigated to evaluate the performance of bentonitic engineered barriers when contacted with alkaline groundwater. Batch and hydraulic conductivity tests were conducted on Na-bentonite using six different alkaline aqueous solutions. For the batch tests, almost no change in the montmorillonite fraction of the bentonite was observed after reacting with alkaline solutions (pH = 8.4–13.1), regardless of the solution type. On the other hand, aluminosilicate minerals (e.g., albite) were dissolved and secondary minerals (e.g., anorthite) were formed in alkaline NaOH solutions (pH > 13). The cation (Ca or Na) concentration primarily affected the swelling properties of bentonite rather than the pH of the solution, which was comparable to the results of the hydraulic conductivity tests. For the Ca solutions, the hydraulic conductivity of the bentonite specimen to the 0.02 mol/L Ca(OH)2 solution (6.5 × 10−9 cm/s) was approximately an order of magnitude lower than that of the bentonite specimen to the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution (5.0 × 10−8 cm/s), whereas the hydraulic conductivity to the 0.02 mol/L Ca(OH)2 + 1 mol/L CaCl2 solution (pH = 11.3) (5.0 × 10−8 cm/s) was slightly higher than that to the 1 mol/L CaCl2 solution (pHi = 8.4) (4.4 × 10−8 cm/s). For the NaOH solutions with pH > 13, the hydraulic conductivity of the bentonite specimen decreased with increasing Na concentration, suggesting that the effect of Na concentration was more dominant than that of permeant pH.



Similar content being viewed by others
References
Ahn HS, Jo HY (2009) Influence of exchangeable cations on hydraulic conductivity of compacted bentonite. Appl Clay Sci 44(1–2):144–150
ASTM (2004a) Standard test methods for measurement of hydraulic conductivity of saturated porous materials using a flexible wall permeameter. D 5084. American Society for Testing and Materials, Philadelphia
ASTM (2004b) Standard test method for swell index of clay mineral component of geosynthetic clay liners. D 5890. American Society for Testing and Materials, Philadelphia
Berner UR (1992) Evolution of pore water chemistry during degradation of cement in a radioactive waste repository environment. Waste Manag 12:201–219
Calábria JAA, Amaral DND, Ladeira ACQ, Cota DSC, Silva TSS (2013) Determination of the cation exchange capacity of bentonite exposed to alkaline fluid. In: 2013 international nuclear atlantic conference
Fernández RF, Máder UK, Rodíguez M, Villa RV, Cuevas J (2009) Alteration of compacted bentonite by diffusion of highly alkaline solutions. Eur J Mineral 21:725–735
Fernández RF, González Ruiz AI, Cuevas J (2014) Nature of C-(A)-S-H phases formed in the reaction Bentonite/Portlandite. J Geochem 2014:1–8
Gates WP, Bouazza A (2010) Bentonite transformations in strongly alkaline solutions. Geotext Geomembr 28:219–225
Gaucher CE, Blanc P (2006) Cement/Clay interactions—a review: experiments, natural analogues, and modeling. Waste Manag 26:776–788
Heikola T, Kumpulainen S, Vuorinen U, Kiviranta L, Korkeakoski P (2013) Influence of alkaline (pH 8.3–12.0) and saline solutions on chemical, mineralogical and physical properties of two different bentonites. Clay Miner 48:309–329
Huertas FJ, Hidalgo A, Rozalén ML, Pellicione S, Domingo C, García-González CA, Andrade C, Alonso C (2009) Interaction of bentonite with supercritically carbonated concrete. Appl Clay Sci 42:488–496
Karnland O, Olsson S, Nisson U, Sellin P (2007) Experimentally determined swelling pressure and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions. Phys Chem Earth 32:275–286
Kaufhold S, Dohrmann R (2010) Stability of bentonites in salt solutions III—calcium hydroxide. Appl Clay Sci 51:300–307
Kaufhold S, Dohrmann R, Koch D, Houben G (2008) The pH of aqueous bentonite suspensions. Clay Clay Miner 56:338–343
Knauss KG, Wolery TJ (1988) The dissolution kinetics of quartz as a function of pH and time at 70°C. Geochim Cosmochim Acta 52:43–53
Lavkulich L (1981) Exchangeable cations and total exchange capacity by the ammonium acetate method at pH 7.0. In: Carter MR (ed) Soil sampling and methods of analysis. Canadian Society of Soil Science, Ottawa, pp 173–175
Meunier A (2005) Clays. Springer, Berlin, p 472
Pusch R, Zwahr H, Gerber R, Schomburg J (2003) Interaction of cement and smectitic clay—theory and practice. Appl Clay Sci 23:203–210
Rhoades J (1982) Soluble salts, methods of soil analysis, part 2. In: Page A, Miller R, Keeney D (eds) Chemical and microbiological properties, 2nd edn. Soil Science Society of America, Madison, pp 167–180
Savage D, Noy D, Mihara M (2002) Modelling the interaction of bentonite with alkaline fluids. Appl Geochem 17:207–223
Savage D, Walker C, Arthur R, Rochelle C, Oda C, Takase H (2007) Alteration of bentonite by alkaline fluids: a review of the role of secondary minerals. Phys Chem Earth 32:287–297
Savage D, Benhow S, Watson C, Takase H, Ono K, Oda C, Honda A (2010) Natural systems evidence for the alteration of clay under alkaline conditions: an example from Searles Lake, California. Appl Clay Sci 47:72–81
Thomas GW (1982) Exchangeable cations. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2. Chemical and microbiological properties—agronomy monograph no. 9, 2nd edn. ASA-SSSA, Madison
Wilson J, Savage D, Bond A, Watson S, Pusch R, Bennett D (2011) Bentonite: a review of key properties, processes and issues for consideration in the UK context. QRS-1378ZG-1, Ver. 1.1
Acknowledgements
This research was supported by the Korean Nuclear Energy R&D program (NRF-2015M2A8A5021871) and the National Research Foundation (NRF-2017R1A2B4008238) of the Ministry of Science, ICT & Future Panning, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anh, H.N., Ahn, H., Jo, H.Y. et al. Effect of alkaline solutions on bentonite properties. Environ Earth Sci 76, 374 (2017). https://doi.org/10.1007/s12665-017-6704-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6704-8