Abstract
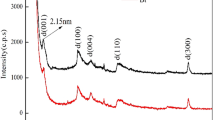
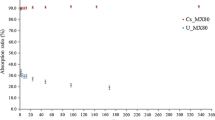
Characterization and adsorption analysis of the bentonite samples were studied by scanning electron microscopy (SEM), energy disperse spectroscopy (EDS), Fourier transformed infrared (FTIR) spectroscopy, and X-ray diffraction (XRD). SEM, EDS, FTIR, and XRD results showed that the ion exchange is one of the adsorption mechanisms of Cs+ onto bentonite and the Al–O–H and H–O–H groups of the bentonite might participate in the Cs+ adsorption process. The effect of the contact time, different concentrations of Cs+, pH value, and bentonite dosage on the adsorption of Cs+ onto bentonite were examined. The study results showed that cesium ions could be effectively adsorbed by bentonite. The adsorption equilibrium was established after 24 h. The distribution coefficient decreased from 1614.57 to 84.93 mL/g, but the adsorption capacity increased from 8.71 to 21.79 mg/g when the initial Cs concentration increased from 100 to 500 mg/L. The distribution coefficient and adsorption capacity for the cesium ions increased with an increase of solution pH in the range of 3 and 11 except for a little decrease at pH 7. The distribution coefficient increased from 66.54 to 3498.62 mL/g, but the adsorptive capacity decreased from 23.48 to 2.86 mg/g with an increasing amount of the adsorbent from 0.25 to 0.5 g. Adsorption isotherms fitted with Freundlich models well. Thermodynamic studies indicated that the sorption capacity of the sorption of cesium ions onto bentonite became weak with a temperature increase and showed the sorption of cesium ions onto bentonite was exothermic.







Similar content being viewed by others
References
Akcanca F, Aytekin M (2014) Impact of wetting-drying cycles on the hydraulic conductivity of liners made of lime-stabilized sand-bentonite mixtures for sanitary landfills. Environ Earth Sci 72(1):59–66
Aksu Z (2002) Equilibrium and kinetic modeling of cadmium (II) biosorption by C. vulgarisin a batch system: effect of temperature. Sep Purif Technol 21:285–294
Basha S, Murthy ZVP, Jha B (2008) Sorption of Hg(II) from aqueous solutions onto Carica papaya: application of isotherms. Ind Eng Chem Res 47:980–986
Galamboš M, Kufčáková J, Rajec P (2009) Adsorption of cesium on domestic bentonites. J Radioanal Nucl Chem 281:485–492
Kang CH, Kim JW, Chun KS, Park JH, Cho WJ, Choi JW, Lee JW, Lee YM (2002) High level radwaste disposal technology development/geological disposal system development. KAERI/RR-2336/2002, Korea Atomic Energy Research Institute, Daejeon
Lee JO, Cho WJ, Choi H (2013) Sorption of cesium and iodide ions onto KENTEX-bentonite. Environ Earth Sci 70(5):2387–2395
Lieser KH, Steinkopff T (1989) Chemistry of radioactive cesium in the hydrosphere and in the geosphere. Radiochim Acta 48:79–86
Macasek F, Navratil J, Dulanska S (2002) Magnetic sorbent for soil remediation—a waste for waste treatment. Sep Sci Technol 37:3673–3691
Mashitah MD, YusAzila Y, Bhatia S (2008) Biosorption of cadmium (II) ions by immobilized cells of Pycnoporus sanguineus from aqueous solution. Bioresour Technol 99:4742–4748
Melkior T, Yahiaoui S, Motellier S, Thoby D, Tevissen E (2005) Cesium sorption and diffusion in Bure mudrock samples. Appl Clay Sci 29:172–186
NCRP (Paperback, April 2007) Cesium-137 in the environment: Radioecology and approaches to assessment and management. NCRP Report No. 154. National Council on Radiation Protection & Measurements, Bethesda, MD
Tolgyessy J, Harangozó M (2000) Radioecology, vol 1, p 14. Matej Bel University, Faculty of Natural Sciences, Banska´ Bystrica
Vejsada J, Hradil D, Řanda Z, Jelínek E, Štulík K (2005) Adsorption of cesium on Czech smectite-rich clays—a comparative study. Appl Clay Sci 30:53–66
Wang XS, Qin Y, Li ZF (2006) Biosorption of zinc from aqueous solutions by rice bran: kinetics and equilibrium studies. Sep Sci Technol 41(4):747–756
Wang JS, Hu XJ, Liu YG, Xie SB, Bao ZL (2010) Biosorption of uranium (VI) by immobilized Aspergillus fumigatusbeads. J Environ Radioact 101:504–508
Ye WM, Wan M, Chen B, Chen YG, Cui YJ, Wang J (2014) An unsaturated hydraulic conductivity model for compacted GMZ01 bentonite with consideration of temperature. Environ Earth Sci 71(4):1937–1944
Acknowledgments
This project is supported by the National Natural Science Foundation of China (Grant No. 11175081), the program of Doctoral Fund of high education (Grant No. 20134324110003), the program of nuclear technology and application of the Key Discipline in Hunan province.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, HJ., Xie, SB., Xia, LS. et al. Study on adsorptive property of bentonite for cesium. Environ Earth Sci 75, 148 (2016). https://doi.org/10.1007/s12665-015-4941-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4941-2