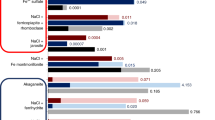
Abstract
Experiments were carried out at 100 bar pressure and 60 or 150 °C in 0.7 m NaCl brine to characterize the reactivity of two Frio quartzofeldspathic sandstone compositions and to elucidate the fate of metals (Ba, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in subsurface reservoirs targeted for carbon dioxide sequestration and storage. The solutions were either acidic (pH ~3) or near-neutral (pH ~8). In the former, acidity resulted from saturation with carbon dioxide (CO2) or by addition of HCl, and in the latter, the pH was attained by addition of NaHCO3. A pair of experiments was conducted without CO2 to trace the behavior of four dissolved metals (Cr, Ni, Pb and Zn) in circum-neutral solutions. The experiments were conducted in rocking autoclave reactors up to 67 days’ time with solutions drawn periodically. Solution analyses indicated modest release of major elements from the starting materials to solution, even at 150 °C. Geochemical modeling indicated supersaturation of the solutions with respect to a variety of Fe- and Mn-bearing phases. Scanning electron microscope (SEM) analyses of the powders both before and after experiments showed evidence for minor dissolution of alkali feldspar, quartz, plagioclase and clay minerals. No evidence for precipitated carbonate phases was found in the CO2-bearing experiments. In general, the concentrations of the metals were below their respective maximum contaminant levels (MCLs) by the end of the experiment, except for Ba, and for Cr and Pb in the experiment in which near-neutral conditions were imposed from the beginning. The data are consistent with metal removal from solution as the pH changes from acidic to neutral and SEM results identified Fe-oxides and sulfides as the likely sinks for Cu, Cr and Zn. The data indicate that even under extreme conditions the likelihood of metal concentrations in drinking water exceeding MCLs through accidental mixing with CO2-bearing solution is very low.











Similar content being viewed by others
References
Bachu S, Gunter WD, Perkins EH (1994) Aquifer disposal of CO2: hydrodynamic and mineral trapping. Energy Convers Manag 35:269–279
Bénezéth P, Palmer DA, Anovitz LM, Horita J (2007) Dawsonite synthesis and reevaluation of its thermodynamic properties from solubility measurements: implications for mineral trapping of CO2. Geochim Cosmochim Acta 71:4438–4455
Benson SM, Cole DR (2008) CO2 sequestration in deep sedimentary formations. Elements 4:325–331
Bruant RG, Celia MA, Guswa AJ, Peters CA (2002) Safe storage of CO2 in deep saline aquifers. Environ Sci Technol 36:240A–245A
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Doughty C, Freifeld BM, Trautz RC (2008) Site characterization for CO2 geologic storage and vice versa: the Frio brine plot, Texas, USA as a case study. Environ Geol 54:1635–1656
Duan ZH, Sun R (2003) An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem Geol 193:257–271
Guyot F, Daval D, Dupraz S, Martinez I, Menez B, Sissmann O (2011) CO2 geologic storage: the environmental mineralogy perspective. C R Geosci 343:246–259
Hovorka SD, Benson SM, Doughty C, Freifeld BM, Sakurai S, Daley TM, Kharaka YK, Holtz MH, Trautz RC, Nance HS, Myer LR, Knauss KG (2006) Measuring permanence of CO2 storage in saline formations: the Frio experiment. Environ Geosci 13:105–121
Kampman N, Bickle M, Wigley M, Dubacq B (2014) Fluid flow and CO2–fluid–mineral interactions during CO2-storage in sedimentary basins. Chem Geol 369:22–50
Karamalidis AK, Torres SG, Hakala JA, Shao H, Cantrell KJ, Carroll S (2013) Trace metal source terms in carbon sequestration environments. Environ Sci Technol 47:322–329
Kaszuba JP, Viswanathan HS, Carey JW (2011) Relative stability and significance of dawsonite and aluminum minerals in geologic carbon sequestration. Geophys Res Lett 38:L08404
Kharaka YK, Cole DR, Hovorka SD, Gunter WD, Knauss KG, Freifeld BM (2006) Gas-water-rock interactions in Frio formation following CO2 injection: implications for the storage of greenhouse gases in sedimentary basins. Geology 34:577–580
Kharaka YK, Thordsen JJ, Hovorka SD, Nance HS, Cole DR, Phelps TJ, Knauss KG (2009) Potential environmental issues of CO2 storage in deep saline aquifers: geochemical results from the Frio-I Brine Pilot test, Texas, USA. Appl Geochem 24:1106–1112
Knauss KG, Copenhaver SA (1995) The solubility of p-xylene in water as a function of temperature and pressure and calculated thermodynamic quantities. Geochim Cosmochim Acta 59:2443–2448
Knauss KG, Dibley MJ, Bourcier WL, Shaw HF (2001) Ti(IV) hydrolysis constants derived from rutile solubility measurements made from 100 to 300 °C. Appl Geochem 16:1115–1128
Little MG, Jackson RB (2010) Potential impact of leakage from deep CO2 geosequestration on overlying freshwater aquifers. Environ Sci Technol 44:9225–9232
Milliken KL (1992) Chemical behavior of detrital feldspars in mudrocks versus sandstones, Frio formation (Oligocene), South Texas. J Sediment Petrol 62:790–801
Milliken KL, McBride EF, Land LS (1989) Numerical assessment of dissolution versus replacement in the subsurface destruction of detrital feldspars, Oligocene Frio formation, South Texas. J Sediment Petrol 59:740–757
Oelkers EH, Cole DR (2008) Carbon dioxide sequestration: a solution to a global problem. Elements 4:305–310
Oelkers EH, Gislason SR, Matter J (2008) Mineral carbonation of CO2. Elements 4:333–337
Saldi GD, Daval D, Morvan G, Knauss KG (2013) The role of Fe and redox conditions in olivine carbonation rates: an experimental study of the rate limiting reactions at 90 and 150 °C in open and closed systems. Geochim Cosmochim Acta 118:157–183
Seyfried WE, Gordon P, Dickson FW (1979) New reaction cell for hydrothermal solution equipment. Am Mineral 64:646–649
Seyfried WE, Janecky DR, Berndt ME (1987) Rocking autoclaves for hydrothermal experiments II. The flexible reaction-cell system. In: Ulmer GC, Barnes HL (eds) Hydrothermal experimental techniques. Wiley, New York, pp 216–239
Shao H, Kukkadapu RK, Krogstad EJ, Newburn MK, Cantrell KJ (2014) Mobilization of metals from Eau Claire siltstone and the impact of oxygen under geological carbon dioxide sequestration conditions. Geochim Cosmochim Acta 141:62–82
Smyth RC, Hovorka SD, Lu J, Romanak KD, Partin JW, Wong C, Yang C (2009) Assessing risk to fresh water resources from long term CO2 injection—laboratory and field studies. Energy Proc 1:1957–1964
Trautz RC, Pugh JD, Varadharajan C, Zheng L, Bianchi M, Nico PS, Spycher NF, Newell DL, Esposito RA, Wu Y, Dafflon B, Hubbard SS, Birkholzer JT (2013) Effect of dissolved CO2 on a shallow groundwater system: a controlled release field experiment. Environ Sci Technol 47:298–305
Wolery TW, Jarek RL (2003) Software user’s manual. EQ3/6, version 8.0. U.S. Dept. of Energy Report, 10813-UM-8.0-00. Sandia National Laboratories, Albuquerque, New Mexico, p 376
Xu T, Kharka YK, Doughty C, Freifeld BM, Daley TM (2010) Reactive transport modeling to study changes in water chemistry induced by CO2 injection at the Frio-I Brine Pilot. Chem Geol 271:153–164
Acknowledgments
This work was supported by the GEO-SEQ program, through the Assistant Secretary for Fossil Energy, Office of Coal and Power Systems through the National Energy Technology Laboratory (Karen Kluger, Program Manager), and by Lawrence Berkeley National Laboratory under Department of Energy Contract No. DE-AC02-05CH11231. We thank Jeff Urban (LBNL) for allowing us to use the ICP-OES, Joern T. Larsen (LBNL) for use of the ICP-MS and April Van Hise and Li Yang for their technical assistance during fluid sample analyses and BET measurements. We also thank Nicholas Pester (LBNL) and Susan Hovorka for insightful reviews of earlier drafts of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Icenhower, J.P., Saldi, G.D., Daval, D. et al. Experimental determination of the reactivity of the Frio Sandstone, Texas, and the fate of heavy metals resulting from carbon dioxide sequestration. Environ Earth Sci 74, 5501–5516 (2015). https://doi.org/10.1007/s12665-015-4560-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4560-y