Abstract
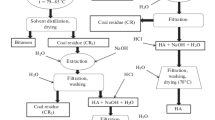
Lignite can be applied directly in natural form on agricultural fields as a soil conditioner. However, there is little information on leaching of risky compounds by its interaction with water. South Moravian lignite was therefore extracted with water at 25 °C and 2.3 % of water-soluble fractions were obtained from lignite corresponding to 0.3 % of total organic carbon. All ten fractions form aromatic and aliphatic structures with oxygen-containing functional groups such as carboxyl groups, alcohols, ethers, esters, can be characterized as fulvic-like and humic-like substances. According to the XPS spectra, the fractions contain two nitrogen forms, one of which is ascribed to pyrroles and the second is related to protonated amines or quaternary nitrogen. Analysis at molecular level showed that the fractions contain compounds such as benzene carboxylic acids and their derivatives, small aliphatic diacids, fatty acids and polyols. Most of the identified molecules reflect clearly the presence of microbial remains in the lignite structure since microbial activity during coalification is well known. The differences between the individual fractions are negligible, especially after 21 days of extraction. From environmental point of view, it seems that the identified compounds do not represent a toxic risk.






Similar content being viewed by others
References
Baes AU, Bloom PR (1990) Fulvic acid ultraviolet-visible spectra: influence of solvent and pH. Soil Sci Soc Am J 54:1248–1254
Berrueta LA, Fernández LA, Vicente F (1991) Fluorescence study of the solubilization of benzo[a]pyrene: application to its detection in coal washing waters. Anal Chim Acta 243:115–119
Cagniant D, Gruber R, Boudou JP, Bilem C, Bimer J, Salbut PD (1998) Structural characterization of nitrogen-enriched coals. Energy Fuel 12:672–681
Chassapis K, Roulia M, Tsirigoti D (2009) Chemistry of metal–humic complexes contained in Megalopolis lignite and potential application in modern organomineral fertilization. Int J Coal Geol 78:288–295
Chen J, LeBoeuf EJ, Dai S, Gu B (2003) Fluorescence spectroscopic studies of natural organic matter fractions. Chemosphere 50:639–647
Cheng L, Zhang R, Bi J (2004) Pyrolysis of a low-rank coal in sub- and supercritical water. Fuel Process Technol 85:921–932
Doskočil L, Pekař M (2012) Removal of metal ions from multi-component mixture using natural lignite. Fuel Process Technol 101:29–34
Doskočil L, Grasset L, Válková D, Pekař M (2014) Hydrogen peroxide oxidation of humic acids and lignite. Fuel 134:406–413
Estournel-Pelardy C, El-Mufleh Al Husseini A, Doskočil L, Grasset L (2013) A two-step thermochemolysis for soil organic matter analysis. Application to lipid-free organic fraction and humic substances from an ombrotrophic peatland. J Anal Appl Pyrol 104:103–110
Fabiańska MJ, Kurkiewicz S (2013) Biomarkers, aromatic hydrocarbons and polar compounds in the Neogenelignites and gangue sediments of the Konin and Turoszów Brown Coal Basins (Poland). Int J Coal Geol 107:24–44
Finkelman RB, Orem W, Castranova V, Tatu CA, Belkin HE, Zheng B, Lerch HE, Maharaj SV, Bates AL (2002) Health impacts of coal and coal use: possible solutions. Int J Coal Geol 50:425–443
Frazier SW, Nowack KO, Goins KM, Cannon FS, Kaplan LA, Hatcher PG (2003) Characterization of organic matter from natural waters using tetramethylammonium hydroxide thermochemolysis GC-MS. J Anal Appl Pyrol 70:99–128
Fuentes M, González-Gaitano G, García-Mina JM (2006) The usefulness of UV–visible and fluorescence spectroscopies to study the chemical nature of humic substances from soils and composts. Org Geochem 37:1949–1959
Godwin J, Manahan SE (1979) Interchange of metals and organic matter between water and subbituminous coal or lignite under simulated coal slurry pipeline conditions. Environ Sci Technol 13:1100–1104
Gorbaty ML, George GN, Kelemen SR (1990) Chemistry of organically bound sulphur forms during the mild oxidation of coal. Fuel 69:1065–1067
Grasset L, Amblès A (1998) Structural study of soil humic acids and humin using a new preparative thermochemolysis technique. J Anal Appl Pyrol 47:1–12
Hänninen K, Niemelä K (1992) Alkaline degradation of peat humic acids. Part II. Identification of hydrophilic products. Acta Chem Scand 46:459–463
Havelcová M, Sýkorová I, Trejtnarová H, Šulc A (2012) Identification of organic matter in lignite samples from basins in the Czech Republic: geochemical and petrographic properties in relation to lithotype. Fuel 99:129–142
Honěk J, Staněk F, Hoňková K, Jelínek J (2009) Coal seams in the South Moravia Lignite Coalfield. Acta Montan Slovaca 14:43–54 (in Czech)
Iordanidis A, Schwarzbauer J, Georgakopoulos A, van Lagen B (2012) Organic geochemistry of amynteo lignite deposit, northern Greece: a multi-analytical approach. Geochem Int 50:159–178
Jelínek J, Staněk F, Vizi L, Honěk J (2011) Evolution of lignite seams within the South Moravian Lignite Coalfield based on certain qualitative data. Int J Coal Geol 87:237–252
Joll CA, Huynh T, Heitz A (2003) Off-line tetramethylammonium hydroxide thermochemolysis of model compound aliphatic and aromatic carboxylic acids: decarboxylation of some ortho- and/or para-substituted aromatic carboxylic acids. J Anal Appl Pyrol 70:151–167
Kashimura N, Hayashi J, Chiba T (2004) Degradation of a Victorian brown coal in sub-critical water. Fuel 83:353–358
Kelemen SR, Gorbaty ML, Kwiatek PJ (1994) Quantification of nitrogen forms in argonne premium. Energy Fuel 8:896–906
Kelemen SR, Freund H, Gorbaty ML, Kwiatek PJ (1999) Thermal chemistry of nitrogen in kerogen and low-rank coal. Energy Fuel 13:529–538
Kelemen SR, Afeworki M, Gorbaty ML, Kwiatek PJ, Sansone M, Walters CC, Cohen AD (2006) Thermal transformations of nitrogen and sulfur forms in peat related to coalification. Energy Fuel 20:635–652
Korshin GV, Li C, Benjamin MM (1997) Monitoring the properties of natural organic matter through UV spectroscopy: a consistent theory. Water Res 31:1787–1795
Kučerík J, Pekař M, Klučáková M (2003) South-Moravian Lignite–potential source of humic substances. Pet Coal 45:58–62
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, Baltimore
Lehtonen T, Peuravuori J, Pihlaja K (2000) Characterisation of lake-aquatic humic matter isolated with two different sorbing solid techniques: tetramethylammonium hydroxide treatment and pyrolysis-gas chromatography/mass spectrometry. Anal Chim Acta 424:91–103
Lehtonen T, Peuravuori J, Pihlaja K (2004) Degradative analysis of aquatic fulvic acid: CuO oxidation versus pyrolysis after tetramethylammonium hydroxide treatments in air and helium atmospheres. Anal Chim Acta 511:349–356
Li A, Hu J, Li W, Zhang W, Wang X (2009) Polarity based fractionation of fulvic acids. Chemosphere 77:1419–1426
Maharaj SVM, Orem WH, Tatu CA, Lerch HE III, Szilagyi DN (2014) Organic compounds in water extracts of coal: links to Balkan endemic nephropathy. Environ Geochem Health 36:1–17
McElmurry SP, Voice TC (2004) Screening methodology for coal-derived organic contaminants in water. Intern J Environ Anal Chem 84:277–287
Milata V, Segľa P (2007) Vybrané kapitoly molekulovej spektroskopie. Slovenská technická uviverzita, Bratislava
Milori DMBP, Martin-Neto L, Bayer C (2002) Humification degree of soil humic acids determined by fluorescence spectroscopy. Soil Sci 167:739–749
Nakajima T, Kanda T, Fukuda T, Takanashi H, Ohki A (2005) Characterization of eluent by hot water extraction of coals in terms of total organic carbon and environmental impacts. Fuel 84:783–789
Nakajima T, Hasegawa H, Nakamata S, Takanashi H, Ohki A (2008) Mutagenicity of eluent by hot water extraction of various coals: effect of chlorination. Fuel 87:3132–3136
Nanny MA, Ratasuk N (2002) Characterization and comparison of hydrophobic neutral and hydrophobic acid dissolved organic carbon isolated from three municipal landfill leachates. Water Res 36:1572–1584
Orem WH, Feder GL, Finkelman RB (1999) A possible link between Balkan endemic nephropathy and the leaching of toxic organic compounds from Pliocene lignite by groundwater: preliminary investigation. Int J Coal Geol 40:237–252
Painter PC, Coleman MM, Jenkins RG, Whang PW, Walker PL (1978) Fourier transform infrared study of mineral matter in coal. A novel method for quantitative mineralogical analysis. Fuel 57:337–344
Pehlivan E, Arslan G (2007) Removal of metal ions using lignite in aqueous solution–Low cost biosorbents. Fuel Process Technol 88:99–106
Pekař (2009) Fluoride anion binding by natural lignite (South Moravian Deposit of Vienna Basin). Water Air Soil Poll 197:303–312
Petrotou A, Skordas K, Papastergios G, Filippidis A (2012) Factors affecting the distribution of potentially toxic elements in surface soils around an industrialized area of northwestern Greece. Environ Earth Sci 65:823–833
Peuravuori J, Pihlaja K (1997) Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal Chim Acta 337:133–149
Peuravuori J, Žbánková P, Pihlaja K (2006) Aspect of structural features in lignite humic acids. Fuel Process Technol 87:829–839
Plaza C, Senesi N, Polo A, Brunetti G, García-Gil JC, D’Orazio V (2003) Soil fulvic acid properties as a means to assess the use of pig slurry amendment. Soil Tillage 74:179–190
Reid MC, Davis JW, Minear RA, Sayler GS (1988) Fulvic acid constituents of coal slurry transport wastewater. Water Res 22:127–131
Rodríguez JF, Schlenger P, García-Valverde M (2014) A comprehensive structural evaluation of humic substances using several fluorescence techniques before and after ozonation. Part I: structural characterization of humic substances. Sci Total Environ 476–477:718–730
Senesi N, Miano TM, Provenzano MR, Brunetti G (1991) Characterization, differentiation, and classification of humic substances by fluorescence spectroscopy. Soil Sci 152:259–271
Song Ch, Schobert HH (1996) Non-fuel uses of coals and synthesis of chemicals and materials. Fuel 75:724–736
Straka P, Marinov S, Tyuliev G (2000) X-ray photoelectron spectroscopy of nitrogen and sulfur functionalities in organic substance of coal. Acta Montana, B 10:36–44
Tan KH (2003) Humic matter in soil and the environment. Dekker, New York
Tanczos I, Rendl K, Schmidt H (1999) The behavior of aldehydes—produced as primary pyrolysis products—in the thermochemolysis with tetramethylammonium hydroxide. J Anal Appl Pyrol 49:319–327
Templier J, Derenne S, Croué JP, Largeau C (2005) Comparative study of two fractions of riverine dissolved organic matter using various analytical pyrolytic methods and a 13C CP/MAS NMR approach. Org Geochem 36:1418–1442
Templier J, Miserque F, Barré N, Mercier F, Croué JP, Derenne S (2012) Is nitrogen functionality responsible for contrasted responses of riverine dissolved organic matter in pyrolysis? J Anal Appl Pyrol 97:62–72
Vieth A, Mangelsdorf K, Sykes R, Horsfield B (2008) Water extraction of coals – potential for estimating low molecular weight organic acids as carbon feedstock for the deep terrestrial biosphere. Geochem 39:985–991
Vlčková Z, Grasset L, Antošová B, Pekař M, Kučerík J (2009) Lignite pre-treatment and its effect on bio-stimulative properties of respective lignite humic acids. Soil Biol Biochem 41:1894–1901
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Wongyai K, Garivait S, Donald O (2013) A geochemistry study of arsenic speciation in overburden from Mae Moh Lignite Mine, Lampang, Thailand. Environ Earth Sci 70:2047–2053
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fert Soils 29:111–129
Zhu Q, Money SL, Russell AE, Thomas KM (1997) Determination of the fate of nitrogen functionality in carbonaceous materials during pyrolysis and combustion using X-ray absorption near edge structure spectroscopy. Langmuir 13:2149–2157
Zsolnay A, Baigar E, Jimenez M, Steinweg B, Saccomandi F (1999) Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 38:45–50
Acknowledgments
This work was supported by project Nr. LO1211, Materials Research Centre at FCH BUT-Sustainability and Development (National Programme for Sustainability I, Ministry of Education, Youth and Sports).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doskočil, L., Grasset, L., Enev, V. et al. Study of water-extractable fractions from South Moravian lignite. Environ Earth Sci 73, 3873–3885 (2015). https://doi.org/10.1007/s12665-014-3671-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3671-1