Abstract
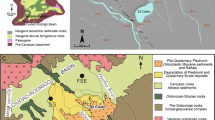
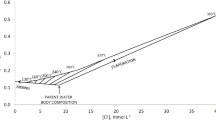
Natural springs have been reliable sources of domestic water and have allowed for the development of recreational facilities and resorts in the Central Appalachians. The structural history of this area is complex and it is unknown whether these natural springs receive significant recharge from modern precipitation or whether they discharge old water recharged over geological times scales. The main objective of this study was to use stable isotopes of water (\(\delta^{18} {\text{O}}_{{{\text{H}}_{2} {\text{O}}}}\) and \(\delta^{2} {\text{H}}_{{{\text{H}}_{2} {\text{O}}}}\)), dissolved inorganic carbon (\(\delta^{13} {\text{C}}_{\text{DIC}}\)) and dissolved sulfate (\(\delta^{34} {\text{S}}_{{{\text{SO}}_{4} }}\) and \(\delta^{18} {\text{O}}_{{{\text{SO}}_{4} }}\)) to delineate sources of water, carbon and sulfur in several natural springs of the region. Our preliminary isotope data indicate that all springs are being recharged by modern precipitation. The oxygen isotope composition indicates that waters in thermal springs did not encounter the high temperatures required for O isotope exchange between the water and silicate/carbonate minerals, and/or the residence time of water in the aquifers was short due to high flow rates. The carbon isotopic composition of dissolved inorganic carbon and sulfur/oxygen isotopic composition of dissolved sulfate provide evidence of low-temperature water–rock interactions and various biogeochemical transformations these waters have undergone along their flow path.






Similar content being viewed by others
References
Andrews JE (2006) Paleo-climatic records from stable isotopes in riverine tufas: synthesis and review. Earth Sci Rev 75:85–104. doi:10.1016/j.earscirev.2005.08.002
Atekwana EA, Fonyuy EW (2009) Dissolved inorganic carbon concentrations and stable carbon isotopes ratios in streams polluted by variable amounts of acid mine drainage. J Hydrol 372:136–148. doi:10.1016/j.jhydrol.2009.04.010
Blasch KW, Bryson JR (2007) Distinguishing sources of ground water recharge by using δ 2H and δ 18O. Ground Water 45:294–308. doi:10.1111/j.1745-6584.2006.00289.x
Bowen GJ (2012) The online isotopes in precipitation calculator, version 2.2. http://www.waterisotopes.org
Clark I, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, Boca Raton, p 311
Claypool GE, Holser WT, Kaplan IR, Sakai H, Zak I (1980) The age curve of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem Geol 28:199–260. doi:10.1016/0009-2541(80)90047-9
Costain JK, Glover L, Sinha AK (1980) Low-temperature geothermal resources in the eastern US. Eos Trans AGU 61:1–3. doi:10.1029/EO061i001p00001
Doctor DH, Kendall C, Sebestyen SD, Shanley JB, Ohte N, Boyer EW (2008) Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream. Hydrol Proc 22:2410–2423. doi:10.1002/hyp.6833
Drever JI (1997) The geochemistry of natural waters: surface and groundwater environments. Prentice Hall Inc., Upper Saddle River
Evans MA, Battles DA (1999) Fluid inclusion and stable isotope analysis of veins from the central Appalachian valley and ridge province: implications for regions synorogenic hydrologic structure and fluid migration. Geol Soc Am Bull 111:1841–1860. doi:10.1130/0016-7606(1999)
Gammons CH, Duaime TE, Parker SR, Poulson SR, Kennelly P (2010) Geochemistry and stable isotope investigation of acid mine drainage associated with abandoned coal mines in Central Montana, USA. Chem Geol 269:100–112. doi:10.1016/j.chemgeo.2009.05.026
Geyh M (2001) Environmental isotopes in the hydrological cycle: principles and application, 39 Volume IV: groundwater saturated and unsaturated zone, IAEA, pp 1–200
Hendy CH (1971) The isotopic geochemistry of speleothems-I. The calculation of the effects of different modes of formation on the isotopic composition of speleothems and their applicability as palaeo-climatic indicators. Geochim Cosmochim Acta 35:801–824. doi:10.1016/0016-7037(71)90127-X
Hobba WA, Fisher DW, Pearson FJ, Chemerys JC (1979) Hydrology and geochemistry of thermal springs of the Appalachians. USGS PP 1044E:1–36
Karim A, Veizer J (2000) Weathering processes in the Indus River Basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem Geol 170:153–177. doi:10.1016/S0009-2541(99)00246-6
Kharaka YK, Berry FAF (1973) Isotopic composition of oil-field brines from Kettleman north Dome, California, and their geologic implications. Geochim et Cosmochim Acta 37:1899–1908
Kharaka YK, Mariner RH (2005) Geothermal systems. In: Gat JR, Froehlich FO, Aggarwal PK (eds) Isotopes in the water cycle. Springer, Netherlands, pp 243–270
Krouse HR, Mayer B (2000) Sulphur and oxygen isotopes in sulphate, in environmental tracers in subsurface hydrology. Academic Publishers, Kluwer, pp 195–231
Kumar P, Das NK, Mallik C, Bhandari RK (2011) Stable isotopes study on geothermal waters in eastern India. Curr Sci 101:1205–1209
Lund JW (1997) Direct heat utilization of geothermal resources. Ren Ener 10:403–408
Manga M. 2012 Springs, Water encyclopedia science and issues, http://www.waterencyclopedia.com/Re-St/Springs.html
Mook WG, Tan FC (1991) Stable carbon isotopes in rivers and estuaries. In: Degens E, Kempe S, Richey J (eds) Biogeochemistry of major world rivers. John Wiley and Sons, New York City, pp 245–264
Moore J (2012) Potential inhibitors and sources of error in the measurement of travertine precipitation rates in a karst stream influenced by thermal mineral waters. MS dissertation, West Virginia University, Morgantown
Mutlu H (2007) Constraints on the origin of the Bahkesir thermal waters (Turkey) from stable isotope (δ 18O, δD, δ 13C, δ 34S) and major-trace element compositions. Turkish J Earth Sci 16:13–32
Pentecost A (2005) Travertine. Kluwer Academic Publishers Group, Dordrecht
Qin D, Turner JV, Pang Z (2005) Hydro-geochemsitry and groundwater circulation in the Xi’an geothermal field, China. Geothermics 34:471–494
Rader EK, Gathright TM. (1984) Stratigraphy and structure in the thermal springs area of the western anticlines, Virginia Division of Natural Resources, pp 13–15
Révész K, Qi H (2006) Determination of the δ(34S/32S) of sulfate in water: RSIL Lab Code1951. In: Methods of the Reston Stable Isotope Laboratory 10, USGS, pp 10–33
Rostron BJ, Holmden C (2000) Fingerprinting formation-waters using stable isotopes, Midale Area, Williston Basin, Canada. J Geochem Expl 69–70:219–223
Seal RR (2003) Stable -isotope geochemistry of mine waters and related solids. In: Jambor JL, Blowes DW, Ritchie AIM (eds). Environmental aspects of mine wastes, mineralogical association of Canada short course, pp 303–334
Sharma S, Sack A, Adams J, Vesper DJ, Capo RC, Hartsock A, Edenborn H (2013) Isotopic evidence of enhanced carbonate dissolution at a coal mine drainage site in Allegheny County, Pennsylvania USA. Appl Geochem 29:32–42. doi:10.1016/j.bbr.2011.03.031
Van Donkelaar C, Hutcheon IE, Krouse HR (1995) δ 34S, δ 18O, δD in Shallow groundwater: tracing anthropogenic sulfate and accompanying groundwater/rock interactions. Water Air Soil Poll 79:279–298
Wittrup MB, Kyser TK, Danyluk T. (1987) The use of stable isotopes to determine the sources of brines in saskatchewan potash mines. In: Gilboy CF, Vigrass LW (eds) Saskatchewan Geological Society Special Publication Number 8 Eco Min of Saskatchewan, pp 159–165
Acknowledgments
This research was performed as part of the collaborative initiative of National Energy Technology Laboratory’s Regional University Alliance (NETL-RUA), under the RES contract DE-FE0004000. J. Moore is acknowledged for help in sampling and sharing some of his thesis geochemical data. The isotopic data presented in the paper is part of bigger ongoing collaborative project with Drs.’ D. Vesper, H. Edenborn, A. Hartsock, R. Capo and B. Stewart. The study design and sampling were made possible by their prior knowledge of these sites. We thank two anonymous reviewers for their comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sack, A.L., Sharma, S. A multi-isotope approach for understanding sources of water, carbon and sulfur in natural springs of the Central Appalachian region. Environ Earth Sci 71, 4715–4724 (2014). https://doi.org/10.1007/s12665-013-2862-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2862-5