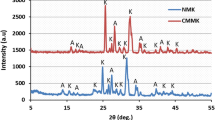
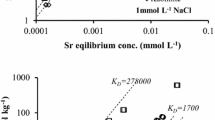
Abstract
The mobility of strontium in subsurface is largely influenced by sorption on to clay minerals. In the present study, kaolinite clay samples collected from the Kalpakkam nuclear plant site were employed to understand the sorption characteristics of strontium by batch method. The effect of several parameters such as time, strontium ion concentration, pH, temperature and ionic strength was investigated. The kinetic studies suggested pseudo-second-order mechanism. The experimental sorption data was fitted to Langmuir adsorption model for obtaining the sorption capacity of the sorbent. The maximum sorption capacity was 5.77 mg/g at 298 K and was found to increase with an increase in temperature. It was observed that the distribution coefficient (K d) of strontium on clay increased as the pH of the solution increased. The distribution coefficient was found to decrease with an increase in concentration of Na+ and Ca2+ ions. This variation of K d suggests that cation exchange is the predominant sorption process. It was also observed that sorption process is endothermic. The thermodynamic parameters such as ∆G 0, ∆H 0 and ∆S 0 were calculated. The negative values obtained for ∆G 0 indicated that the sorption of strontium on clay was spontaneous at all studied concentrations. ∆G 0 becomes more negative with an increase in temperature, suggests that the sorption process is more favorable at higher temperatures.











Similar content being viewed by others
References
Adeleye SA, Clay PG, Oladipo MOA (1994) Sorption of caesium and europium ions on clay minerals. J Mater Sci 29:954–958
Akar D, Shahwan T, Eroglu AE (2005) Kinetic and thermodynamic investigations of strontium ions retention by natural kaolinite and clinoptilolite minerals. Radiochim Acta 93:477–485
Bascetin E, Atun G (2006) Adsorption behavior of strontium on binary mineral mixtures of montmorillonite and kaolinite. Appl Radiat Isot 64:957–964
Borah D, Satokawa S, Kato S, Kojima T (2009) Sorption of As (V) from aqueous solution using acid modified carbon black. J Hazard Mater 162:1269–1277
Brockett TW, Placak OR (1953) Removal of radioisotopes from waste solutions by soils–soils studies with Conasauga Shale. Proc of Eighth Ind Waste Conf, pp 393–409
Buddemeier RW, Hunt JR (1988) Transport of colloidal contaminants in groundwater: radionuclide migration at the Nevada test site. Appl Geochem 3:535–548
Bunde RL, Rosentreter JJ, Liszewski MJ, Hemming CH, Welhan J (1997) Effects of calcium and magnesium on strontium distribution coefficients. Environ Geol 32:219–229
Deepthi Rani R, Sasidhar P (2010) Stability assessment and characterization of colloids in coastal groundwater aquifer system at Kalpakkam. Environ Earth Sci 62:233–243
Deepthi Rani R, Sasidhar P (2011) Physico-chemical characterization of clays at Kalpakkam nuclear plant site towards assessment of radioactive waste disposal. Nucl Eng Des 241:2353–2358
Degueldre C, Baeyens B, Goerlich W, Riga J, Verbist J, Stadelmann P (1989) Colloids in water from a subsurface fracture in granitic rock, Grimsel Test Site, Switzerland. Geochim Cosmochim Acta 53:603–610
Dogan M, Alkan M (2003) Removal of methyl violet from aqueous solution by perlite. J Colloid Interf Sci 267:32–41
Erdal BR, Daniels WR, Thompson JL, Aguilar RD, Bayhurst BP, Duffy CJ, Lawrence FO, Maestas S, Oliver PQ, Wolfsberg K (1978) Laboratory studies of radionuclide distributions between selected groundwaters and geologic media: sorption–desorption studies on Granite. WISAP, PNL-SA-7352, Richland
Erdal BR, Aguilar RD, Bayhurst BP, Daniels WR, Duffy CJ, Lawrence FO, Maestas S, Oliver PQ, Wolfsberg K (1979a) Sorption–desorption studies on granite. LA-7456-MS, Los Alamos
Erdal BR, Bayhurst BP, Daniels WR, DeVilliers SJ, Lawrence FO, Thompson JL, Wolfsberg K (1979b) Parameters affecting radionuclide migration in Argillaceous media. OECD/NEA, Paris
Fan Q, Shao D, Lu Y, Wu W, Wang X (2009) Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni (II) to Na–attapulgite. Chem Eng J 150:188–195
Galambos M, Kufcakova J, Rajec P (2009) Sorption of strontium on Slovak bentonites. J Radioanal Nucl Chem 281:347–357
Gokturk H, Eylem C, Hatipoglu S, Erten HN (1995) Radiochemical studies of the sorption behavior of strontium and barium. J Radioanal Nucl Chem 198:449–456
Gschwend PM, Reynolds MD (1987) Monodisperse ferrous phosphate colloids in an anoxic groundwater plume. J Contam Hydrol 1:309–327
Hening C, Reich T, Dahn R, Scheidegger AM (2002) Structure of uranium sorption complexes at montmorillonite edge sites. Radiochim Acta 90:653–657
Heremans R, Bonne A, Manfroy P (1981) Experimentation on and evaluation of near-field phenomena in clay, The Belgian approach. OECD/NEA, Paris
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Jeong CH (2001) Mineralogical and hydrochemical effects on adsorption removal of Cs-137 and Sr-90 by kaolinite. J Environ Sci Health A Toxicol Hazard Subst Environ Eng 36:1089–1099
Karasyova ON, Ivanova LI, Lakshtanov LZ, Lövgren L (1999) Strontium sorption on hematite at elevated temperatures. J Colloid Interf Sci 220:419–428
Kenna BT (1980) Temperature and pH effects on sorption properties of subseabed clay. Scientific basis for nuclear waste management, vol 3. Pergamon Press, New York
Krouglov SV, Kurinov AD, Alexakhin RM (1998) Chemical fraction of 90Sr, 106Ru, 137Cs and 144Cs in Chernobyl-contaminated soils: an evolution in the course of time. J Environ Radioact 38:59–76
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Liang TJ, Hsu CN, Liou DC (1993) Modified Freundlich sorption of cesium and strontium on Wyoming bentonite. Appl Radiat Isot 44:1205–1208
Lu N, Mason CFV (2001) Sorption–desorption behaviour of strontium-85 onto montmorillonite and silica colloids. Appl Geochem 16:1653–1662
Ma C, Eggleton RA (1999) Cation exchange capacity of kaolinite. Clays Clay Miner 47(2):174–180
McHenry JR, Rhodes DW, Rowe PP (1956) Chemical and physical reactions of radioactive liquid wastes with soils. USAEC, TID-7517
Meyer RE (1979) Systematic study of nuclide adsorption on selected geologic media. ONWI Report
Nandi BK, Goswami A, Purkait MK (2009) Adsorption characteristics of brilliant green dye on kaolin. J Hazard Mater 161:387–395
Papachristodoulou CA, Assimakopoulos PA, Gangas NHJ (2002) Strontium adsorption proprieties of aluminium-pillared montmorillonite carrying carboxylate functional groups. J Colloid Interf Sci 245:32–39
Parkman RH, Charnock JM, Livens FR, Vaughan DJ (1998) A study of the interaction of strontium ions in aqueous solution with the surfaces of calcite and kaolinite. Geochim Cosmochim Acta 62:1481
Pusch R (1992) Use of bentonite for isolation of radioactive-waste products. Clay Miner 27:353
Pusch R (1999) Clay colloid formation and release from MX-80 buffer. SKB Technical Report TR 99-31
Sahai N, Caroll SA, Roberts S, O’Day PA (2000) X-ray adsorption spectroscopy of strontium (II) coordination, II. Sorption and precipitation at kaolinite, amorphous silica and goethite surfaces. J Colloid Interf Sci 222:198–212
Sasidhar P (1993) Safety assessment of low level radioactive waste disposal facility at Kalpakkam. PhD Thesis, Anna University
Sheha RR, Metwally E (2007) Equilibrium isotherm modeling of cesium adsorption onto magnetic materials. J Hazard Mater 143:354–361
Sheng G, Wang S, Hu J, Lu Y, Li J, Dong Y, Wang X (2009) Adsorption of Pb(II) on diatomite as affected via aqueous solution chemistry and temperature. Colloid Surf A Physicochem Eng Aspects 339:159–166
Singh VS, Saxena VK, Jain SC, Anjaneyulu GR, Prakash BA, Mondal NC (2003) Hydrogeological and geophysical investigations at PFBR site, Kalpakkam, Tamilnadu. Tech Rept No NGRI-2003-GW-396
Tan KH (1998) Principles of soil chemistry. Marcel Dekker Inc, New York
Tan KH (2000) Environmental soil science. Marcel Dekker Inc, New York
Tekin N, Kadinci E, Demirbas O, Alkan M, Kara A (2006) Adsorption of polyvinylimidazole onto kaolinite. J Colloid Interf Sci 296:472–479
Tsai SC, Juang KW (2000) A comparison of linear and nonlinear forms of isotherm models for strontium sorption on a sodium bentonite. J Radioanal Nucl Chem 243:741–746
Um W, Papelis C (2003) Sorption mechanisms of Sr and Pb on zeolitized tuffs from the Nevada test site as a function of pH and ionic strength. Am Miner 88:2028–2039
Westrich HR, Brady PV, Cygan RT, Nagy KL, Anderson HL (1997) Metal sorption on kaolinite. http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=463578
Xu D, Chen C, Tan X, Hu J, Wang X (2007) Sorption of Th(IV) on Na-rectorite: effect of HA, ionic strength, foreign ions and temperature. Appl Geochem 22:2892–2906
Xu D, Zhou X, Wang X (2008) Adsorption and desorption of Ni2+ on Na-montmorillonite: effect of pH, ionic strength, fulvic acid, humic acid and addition sequences. Appl Clay Sci 39:133–141
Zhang ML, Ren A, Shao D, Wang X (2006) Effect of fulvic acid and ionic strength on the sorption of radiostrontium on Chinese calcareous soil and its solid components. J Radioanal Nucl Chem 268:33–36
Acknowledgments
One of the authors, Ms. Deepthi Rani, gratefully acknowledges the research fellowship extended by Atomic Energy Regulatory Board (AERB), Government of India, Niyamak Bhavan, Mumbai, to pursue the present investigations. The authors gratefully acknowledge the help rendered by Chemistry Group of Indira Gandhi Centre for Atomic Research (IGCAR) for analysis using Atomic Absorption Spectroscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deepthi Rani, R., Sasidhar, P. Geochemical and thermodynamic aspects of sorption of strontium on kaolinite dominated clay samples at Kalpakkam. Environ Earth Sci 65, 1265–1274 (2012). https://doi.org/10.1007/s12665-011-1374-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1374-4