Abstract
Hyperspectral plant signatures can be used as a short-term, as well as long-term (100-year timescale) monitoring technique to verify that CO2 sequestration fields have not been compromised. An influx of CO2 gas into the soil can stress vegetation, which causes changes in the visible to near-infrared reflectance spectral signature of the vegetation. For 29 days, beginning on July 9, 2008, pure carbon dioxide gas was released through a 100-m long horizontal injection well, at a flow rate of 300 kg day−1. Spectral signatures were recorded almost daily from an unmown patch of plants over the injection with a “FieldSpec Pro” spectrometer by Analytical Spectral Devices, Inc. Measurements were taken both inside and outside of the CO2 leak zone to normalize observations for other environmental factors affecting the plants. Four to five days after the injection began, stress was observed in the spectral signatures of plants within 1 m of the well. After approximately 10 days, moderate to high amounts of stress were measured out to 2.5 m from the well. This spatial distribution corresponded to areas of high CO2 flux from the injection. Airborne hyperspectral imagery, acquired by Resonon, Inc. of Bozeman, MT using their hyperspectral camera, also showed the same pattern of plant stress. Spectral signatures of the plants were also compared to the CO2 concentrations in the soil, which indicated that the lower limit of soil CO2 needed to stress vegetation is between 4 and 8% by volume.
Similar content being viewed by others
Introduction
With the effects of climate change on the rise, reduction of the amount of CO2 released into the atmosphere is crucial. Injection of CO2 into deep underground formations, known as geologic carbon sequestration, can be an effective method of mitigation by preventing the CO2 injected into underground sequestration formations from entering the atmosphere. In order for geologic sequestration to be successful, methods of verification are important to maintain the sequestered CO2 and to assure the public that the sequestration operation is safe. It is therefore important to develop techniques for long-term CO2 leak detection at the surfaces of the CO2 sequestration fields (Pickles and Cover 2005).
Analyzing hyperspectral plant signatures over CO2 sequestration fields can confirm that the sequestration fields have not been compromised. If a leak were to occur, the excess amount of CO2 in the top layers of soil near the surface would stress the vegetation above the sequestration field, which can be seen as changes in their spectral signatures. CO2-induced stress has been recognized in the spectral signature of plants over volcanic CO2 vents at the Latera (Bateson et al. 2008) and Long Valley (De Jong 1996; Hausback et al. 1998; Martini et al. 2000) calderas, as well as in laboratory experiments (Noomen and Skidmore 2009). Conversely, if vegetation over a sequestration field has healthy spectral signatures, it would indicate that CO2 is being sequestered effectively. Because the basic requirement of this technique is just the presence of healthy vegetation over the sequestration field, hyperspectral plant signatures are particularly useful tool for monitoring sequestration fields for years to centuries (Pickles and Cover 2005).
This technique was studied during a shallow underground leak experiment held at Montana State University (MSU) in Bozeman, Montana. Pure CO2 gas was released for 29 days from a 100-m long, horizontal CO2 injection well, located about 1–2.5 m underground. During this time, the health of vegetation over the west end of the injection well was determined by measuring their hyperspectral reflectance signatures with a field spectrometer. In addition, airborne hyperspectral imagery was acquired from a low-flying aircraft using a hyperspectral camera system developed by Resonon, Inc. of Bozeman, MT (www.resonon.com). The spectral signatures of the plants were also correlated with variations in soil CO2 concentrations measured directly over, and at 2.5, 5, 7.5, and 10 m from the well by Lewicki et al. (2009, this volume).
Plant stress and spectral signatures
The term “plant stress” tends to be used in numerous ways because monitoring plant health has many applications. In a general sense, plant stress occurs when environmental conditions are unfavorable for optimal plant growth. Some of the conditions that can cause stress are drought, extreme heat or cold, insect infestation, waterlogging, bacterial diseases, oxygen depletion, nutrient deficiencies, or acidic soil (Lichtenthaler 1998). Although excess CO2 has also been shown to negatively affect plants, the exact mechanism(s) by which it harms vegetation is not known. Most likely, the CO2 gas displaces oxygen to the roots of the plant, which occurs in natural gas leaks (Noomen et al. 2008). Other possibilities are that large amounts of CO2 could change the pH and redox potential of soil or alter natural microbial environments (Noomen et al. 2006). The result of vegetation experiencing stress over long periods can be stunted growth, reduced water content, or a decrease in leaf chlorophyll concentrations. This can lead to replacement by other more tolerant plant species which then becomes habitat modification (Pickles and Cover 2005). We used the decrease in chlorophyll concentrations as an indicator of plant stress because it is a typical response regardless of species of vegetation or cause of stress (Carter 1993). In addition, chlorophyll can be readily estimated in the reflectance spectra of the vegetation (Carter and Knapp 2000; Hill 2004; Zhang et al. 2008; Moorthy et al. 2008).
When light contacts a leaf, the various wavelengths can be absorbed, reflected, or transmitted based on the leaf’s chemical and physical structure. This interaction results in a distinctive spectrum of reflected light. Healthy plants tend to have relatively low reflectance in the visible (~400 to 720 nm) with a peak in the green range (~550 nm) and high reflectance in the near-infrared (NIR) (~700–1,400 nm). The spectral signature of plants in the visible is caused by the various pigments they contain, such as carotenoid and chlorophyll compounds. Chlorophyll gives a healthy plant its green color because chlorophyll reflects light in the green and absorbs light elsewhere in the visible (Blackburn 2007). Specifically, chlorophyll causes a strong absorption feature in healthy plant spectra from approximately 600 to 700 nm. The size and detailed shape of this absorption feature have been found to correlate with the amount of chlorophyll in a plant because a decrease in the amount chlorophyll would also decrease the plant leaf’s overall absorptivity in the range of the absorption feature (Carter 1993, Carter and Knapp 2001; Smith et al. 2004; Noomen et al. 2006; Noomen and Skidmore 2009). Therefore, if a particular plant is stressed by excess CO2 from a faulty sequestration field, the chlorophyll concentrations will decrease, and the chlorophyll absorption feature of its spectral signature will change accordingly.
Even though hyperspectral signatures can be used to estimate the amount of plant stress, it is unable to distinguish between the different causes of stress. This uncertainty can be problematic when testing for CO2 leaks. A sequestration field could appear unhealthy spectrally, but the stress could be caused by something unrelated to escaping CO2. To reduce the number of possible false positives, it is important to normalize measurements by comparing plant spectra to identical environmental conditions outside the sequestration field. Normalization can help determine if the identified stress is seasonal (for example, high summer heat), which would affect vegetation regionally. The spatial distribution of stressed vegetation may also yield information about the pathways by which CO2 migrates from depth to the surface. In addition, knowing the location of CO2 pipelines and other potential leak sites would be extremely useful since the CO2 stress would probably be located near by. Thus, hyperspectral plant signatures could still be used to verify that the sequestration fields have not leaked (with healthy spectral signatures across the field) or at least focus ground-based CO2 monitoring techniques on potential leak sites.
Methods
Field design
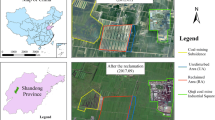
In a MSU agricultural field, a 100-m long, horizontal CO2 injection well was installed at depth varying between approximately 1 and 2.5 m. Pure CO2 gas was injected for 29 days from July 9 to August 7, 2008 at a flow rate of 300 kg day−1 (Spangler et al. 2009, this volume). Prior to the start of the injection, a 20- by 30-m patch of vegetation, centered over the west end of the horizontal injection well, was selected for the plant stress experiment. This section of the field was left unmown except for access paths and was not disturbed for the duration of the experiment (Fig. 1). Within the plant study area, 68 sites were chosen where plant spectral measurements were taken daily. Measurements started 2 days before the start of the injection until 1 week after the CO2 injection was shut off (July 5 to August 11, 2008). Measurement sites ranged in position from directly over the injection well out to 10 m from the well horizontally. In addition, 24 measurement sites were located at 1-m intervals directly above the injection well (Fig. 2). This array gave plant reflection spectra both within and outside of the injected CO2 leak zones so the observations could be normalized for other environmental factors affecting the plants. The plant species within the field consisted primarily of various species of short and tall grasses, alfalfa, dandelions, and a variety of clovers. Each measurement site contained random mixes of vegetation species. Table 1 contains a full list of plant types with their scientific names that were identified on July 30, 2008. This list may not be complete as some species may have been dormant at the time.
Field spectrometer measurement techniques
Plant spectral signatures were measured using a “FieldSpec Pro” spectrometer by ASD, Inc., (www.asdi.com). It was a full range spectrometer that measured reflectance spectra from 350 to 2,500 nm with a 1-nm bandwidth. The spectrometer was connected to a fiber optic cable fitted with a 5° lens. The lens was held approximately 1 m above the ground from a nadir position, which resulted in the spectrometer having a field-of-view of approximately 10 cm. To reduce noise in the spectra, the spectrometer was programed to give a final spectral measurement that was the average of 100 spectra taken continuously at each site. This spectrometer was also designed to self-regulate many of the settings. To optimize this feature, the lens of the spectrometer was held over the vegetation to be measured for approximately 3 s prior to acquiring each spectral signature. This time allowed the spectrometer to self-adjust its settings before taking each measurement. Precautions were also made not to expose the lens of the spectrometer to extreme bright light or darkness. These techniques appeared to reduce noise and increased the reproducibility of the data significantly.
Several procedures were used to eliminate illumination and atmospheric factors that would affect the data. A “Spectralon” white “reflectance plate” provided the solar reference spectrum used to calculate reflectance spectra from the raw collected spectra. A white plate reference spectrum was measured before collecting any plant signatures and re-measured it after every few spectral readings. In addition, spectral readings were only acquired under cloud-free skies with minimal haze. Each day, data were collected in the same sequence as close to solar noon as possible, typically between 9:30 am and 1:30 pm. This approach gave a consistent solar intensity and angle at each measurement site throughout the experiment.
Classification of hyperspectral plant signatures
To aid in analyzing patterns of plant stress, the hyperspectral plant spectra were classified using the software program ENVI 4.5 (www.ittvis.com). As a first step, we created a computer program to compile the individual spectra recorded by the FieldSpec Pro spectrometer into data cubes, thereby creating artificial images of the plant study area. The artificial images show the discrete spectral measurements taken on a given day with their true relative orientations. The images have a 0.5-m pixel size. Each pixel contains either the spectra measured by the FieldSpec Pro at that site or were assigned a spectrum with zero reflectance and were ignored in subsequent analysis. These artificial images were created for each day of the experiment. They are oriented with the east edge of the plant study area at the top of the artificial image with the CO2 injection well running along the center of the image (Fig. 3).
Each artificial image of the field was processed with an ENVI classification tool called Spectral Angle Mapper (SAM). This method classifies measured spectra to reference endmember spectra based on how closely the observed and reference spectra compare (Kruse et al. 1990). This technique has been found to be relatively insensitive to illumination effects caused by atmospheric conditions or differences in leaf angle relative to the spectrometer lens line of sight (Sohn and Rebello 2002).
The SAM classification utilized more of the spectral signature than other simple indices of plant health, which may only compare reflectance at select wavelengths. The supervised SAM classification was based on spectral signatures from 350 to 800 nm. The spectra were divided into three classes: Healthy Vegetation, Moderate to Highly Stressed Vegetation, and Extremely Stressed Vegetation. A total of nine spectra were chosen as endmembers for the SAM classification, with four endmembers as “Healthy Vegetation”, two as “Moderately to Highly Stressed Vegetation”, and three as “Extremely Stressed Vegetation”. Endmembers were based on the overall shape of the spectra, variety of vegetation, quality of spectral signal, and on the depth of their chlorophyll absorption feature from approximately 600 to 700 nm. The depth of this absorption feature was estimated by subtracting the maximum reflectance, between 525 and 575 nm, from the minimum reflectance, between 675 and 700 nm. A relatively large absorption depth was considered to be “Healthy Vegetation,” whereas an absorption depth very close to zero (either positive or negative) was “Moderate to Highly Stressed Vegetation” and a large negative absorption value was “Extremely Stressed Vegetation”. While measuring the depth of this chlorophyll absorption feature gives an estimation of stress level, it was not used as a final classifier because we wanted to utilize the entire spectral signature. Figure 4 shows representative endmember spectra for each class.
Aerial hyperspectral imagery
On August 5, 2008 (after 27 days of CO2 injection), a hyperspectral image was acquired of the entire injection well from a low-flying aircraft. The spectrometer, developed by Resonon, Inc. of Bozeman, MT, had a spectral range of 400–880 nm with a wavelength resolution of approximately 6 nm. ENVI’s supervised classification tool with Spectral Angle Mapper was used to analyze the image over the spectrometer’s full spectral range. Reference endmember spectra were determined on the basis of spectral shape, particularly the depth of the chlorophyll absorption feature, as well as on plant species. The image was classified into five categories: High stress, Moderate stress, Low or Seasonal Stress, Healthy Vegetation (grasses), and Healthy Vegetation (herbaceous legumes). Figure 5 is the hyperspectral imagery shown in true color.
Results
By comparing changes in shape of the hyperspectral plant signatures, stress could be identified in some of the plants close to the well after 4–5 days of CO2 injection (Fig. 6). After 14 days, the spatial extent of plant stress reached a maximum with two discrete, identifiable zones of plant stress. One zone was located over the injection well, along the eastern edge of the vegetation study area. It extended out to at least 2.5 m on the north side of the injection well and out to approximately 1 m on the south side. The second zone of plant stress was in the center of the vegetation study area approximately 20 m from the east edge. It was also located over the injection well and extended out to at least 2.5 m in all directions.
Because hyperspectral plant signatures are very sensitive to environmental changes, this method indicated plant stress using before changes in vegetation were visible to the eye. Large decreases in chlorophyll can make vegetation appear more yellow or brown (Fig. 7). However, changes in the spectral signature can indicate chlorophyll decreases even when there are only small deceases in chlorophyll and the vegetation is still green. In addition, the amount of stress measured during the experiment seemed to be species dependent. The tall grasses and alfalfa in the field reacted quickly, whereas dandelion and some naturally short grasses appeared to be more resistant to stress.
Spectral measurements were collected for 1 week past the end of the injection. It was unclear whether the extremely stressed vegetation began to recover during this time. It was likely that the prolonged exposure to the high amount of CO2 applied enough stress to irreparably damage the vegetation. However, new vegetation, specifically orchard grass and dandelions, began to grow anew in zones of extreme plant stress, which may suggest that certain species of vegetation are more capable of growing under CO2 stress.
Classification results: field spectrometer artificial images
The classification from the artificial images of the field also shows two locations of plant stress by the end of the CO2 injection: (1) on the east edge and (2) in the center of the plant study area. Figure 8a–f is the classification images at the start of the injection, at 10, 14, 16, and 23 days of injection, and 1 day following the end of the injection, respectively. According to the classifications, plants began to experience moderate to high amounts of stress after approximately 6 days of the injection. The size and intensity of the plant stress zones increased throughout the duration of the injection. Near the end of the experiment (early August), the entire field appears to be stressed to some degree (Fig. 8f), which was due to natural changes in the grasses lifecycle and/or by extreme weather (for example, excessive heat or the hail storm on July 22, 2008). Despite seeing this trend of increasing stress across the field, the pattern of two zones of extreme stress is still identifiable. This is an additional benefit for CO2 sequestration monitoring, because it shows that stress from an influx of CO2 can be distinguished from seasonal or other environmental stressors.
Classifications of artificial image using ENVI Spectral Angle Mapper algorithms. Green Healthy vegetation, orange moderately to highly stressed vegetation, red extremely stressed vegetation. a Before injection (July 9, 2008); b after 10 days (July 19, 2008); c after 14 days (July 23, 2008); d After 16 days (July 25, 2008); e 23 days (August 1, 2008); f 1 day after end of the 29-day injection (August 8, 2008)
Classification results: aerial hyperspectral imagery
Figure 9 is the SAM classification of the hyperspectral imagery, which was acquired after 27 days of CO2 injection (August 5, 2008). The classification figure (Fig. 9) also shows the two same zones of plant stress, each measuring approximately 2 m in diameter. Similar-sized zones of plant stress are also found along the length of the entire injection well. These other zones were in mown grass that was approximately 15 cm tall. This demonstrates how this monitoring technique was capable of detecting CO2 leaks over areas with different types of vegetation. One zone of plant stress is difficult to distinguish at the north end of the injection well (Fig. 9). It appears similar to zones of low plant stress caused by frequent foot traffic. However, its location directly over the injection well suggests that the plant stress is connected to the CO2 injection. There are also areas that have been classified as high plant stress that were actually caused by disturbances from equipment from other participants of this experiment that can be seen in the true color aerial image (Fig. 8a). Comparing the locations of plant stress with locations of potential leak site (pipelines, for example) and artificial structures helped indentify the various causes of plant stress.
Classification of Aerial Hyperspectral Imagery acquired by Resonon Inc. Classification was performed using ENVI Spectral Angle Mapper algorithms. White boxes show locations of areas of apparent plant stress caused by equipment from outside experiments. The red circle indicates location of the subtle fifth zone of plant stress caused by the injected CO2
In addition to identifying zones of plant stress, different types of vegetation could be distinguished in the image. Because the tall grasses of the plant study area were left unmown, there were dried seeds on top of the grass stalks. Although that was normal for those species of grass during late summer, it made the unmown area appear slightly stressed. This effect occurs because the aerial spectrometer looks vertically onto the vegetation, seeing mainly the dried seed pods. Outside of the plant study area, the vegetation had been mown prior to the start of the experiment and fresh and healthy vegetation was growing in. This image also demonstrates the effect of plant structure on the hyperspectral signature. Across the MSU agricultural site, the vegetation is typically some variety of either grass or herbaceous legume (Alfalfa, clover, or bird’s foot trefoil). Even if both categories of vegetation are considered healthy, their spectral signatures may be distinct because of the differences in their physical leaf structures, specifically vertical blades of grass compared to more horizontal wide leaves. These differences in spectral signatures were identified using ENVI’s classification algorithms. It is also important to note that these effects from leaf structure are mostly pronounced outside the chlorophyll absorption feature and do not significantly affect the ability to detect stress from the spectral signatures.
Associated observations
Soil CO2 fluxes
Soil CO2 fluxes were measured using the accumulation chamber method on a grid repeatedly on a daily basis during the injection by Lewicki et al. (2009, this volume). Figure 10 shows an example of a map of log soil CO2 flux, interpolated based on measurements made on August 1, 2008 (23 days of CO2 injection). The location of the plant study area is shown for reference. Five separate zones of relatively high CO2 flux can be seen, which are approximately 2–3 orders of magnitude above background levels. They are roughly circular to elliptical in shape ranging from approximately 5 to 15 m in diameter. Within the boundaries of the plant study area, there are two zones of high CO2 flux, located in the center of the plant study area and at the eastern edge.
Map of log soil CO2 flux, interpolated based on measurements made at the black dots [modified from Lewicki et al. 2009, (this volume)]. The blue rectangle represents the CO2 injection well. The red rectangle marks the plant study area. White squares are soil CO2 concentration probes. Note the two zones of high CO2 flux at the east edge and in the center of the plant study area
Soil CO2 fluxes were also measured at 1-m intervals along the surface trace of the injection well on July 14, 2008 (5 days of CO2 injection) by Lewicki et al. (2009, this volume). Figure 11 shows the same five zones of high flux along the injection well, two of which were located within the plant stress area. One high CO2 flux zone, located in the center of the plant study area, is approximately 6 m in diameter and has a maximum flux of approximately 3,700 g m−2 day−1. The other maximum, at 6,500 g m−2 day−1, is located 2 m northeast of the eastern edge of the plant study area, while CO2 flux is about 700 g m−2 day−1 at the edge of the plant study area.
Soil CO2 fluxes measured along the surface trace of the horizontal well [modified from Lewicki et al. 2009, (this volume)]. The area of the plant study area is indicated by the shaded region
Soil CO2 concentrations
Soil CO2 concentrations were measured at 30 cm depth at distances of 0, 2.5, 5, 7.5 and 10 m northwest of the injection well (Fig. 9) using non-dispersive infrared gas analyzers every 1 s and averaged over half-hour periods (Lewicki et al. 2009, this volume) (see Figs. 2, 10 for locations). Figure 12 shows time series of the soil CO2 concentrations. Over the injection well, soil CO2 concentrations rose to approximately 13–14% CO2 by volume. The plants over the injection well also began to show stress after 4 days. CO2 concentrations measured 2.5 m from the well ranged from 6 to 10%, averaging approximately 8% CO2 by volume. Nearby plants showed stress after 14 days. At 5 m from the well, soil CO2 concentrations rose to a maximum of 3–4%. At 7.5 and 10 m from the injection well, soil CO2 concentrations did not rise significantly during the injection, staying close to 1–2% CO2 by volume. None of the plants at 5, 7.5, or 10 m showed significant signs of stress. From these observations, the lower limit of the CO2 concentration needed in the soil to begin to stress vegetation could be inferred. Because plants at 2.5 m were stressed, but those at 5 m were not, the lower limit of soil CO2 concentration can be estimated to be between 4 and 8% by volume, which was approximately 3–4 times greater than background levels. Bateson et al. (2008) performed a leak detection experiment over natural volcanic CO2 vents in Italy by monitoring plant health using various remote sensing techniques. The minimum soil CO2 concentration of their detected gas vents was 5.6% which falls within the range determined in this experiment. In a greenhouse experiment on maize plants, Noomen and Skidmore (2009) found a correlation between high percentages of CO2 in soil and decreases in chlorophyll content and plant growth. However, 50% CO2 was the minimum CO2 concentration to give statistically different results.
Time series of soil CO2 concentrations [Lewicki et al. 2009, (this volume)]. Soil probes were located at east edge of plant study area at varying distances from CO2 injection well
Discussion
Everywhere high CO2 flux was measured, it was associated with a corresponding zone of plant stress. This result shows the potential of using hyperspectral plant signatures for CO2 leak detection. Both the field spectrometer and the airborne hyperspectral imager measured the same two discrete zones of plant stress, on the eastern edge and in the center of the plant study area. These zones coincided with areas of high measured CO2 flux. The airborne hyperspectral imagery also shows three additional zones of plant stress outside the plant study area, whose locations match the zones of high CO2 flux measured along the entire injection well (Fig. 9). The high similarity between the distinctive distributions of extremely stressed vegetation and high CO2 flux indicated that injected CO2 is the cause of the plant stress.
One difficulty of this leak detection method is in the ability to recognize stress caused by excess CO2 independent of other environmental stressors. However, the spatial distribution of relative stress across a sequestration field can help to identify the possible causes for the identified plant stress. In this experiment, two zones of plant stress were located that were focused and sharply delineated from the rest of the field in circular patterns that were centered over the leaks in the injection well. This pattern would not be expected for stress caused by weather, infestation, or poor soil which would act on a regional scale. In addition, the size of plant stress zones remained consistent during changing environmental conditions. For instance, several days of consistent rainfall did not appear to alleviate stressed vegetation, indicating that dehydration was not the cause of the plant stress. The signal of extreme plant stress was also distinct enough not to be clouded by seasonal stress at the end of the summer, showing that seasonal stress was not the only stressor present. The link between injected CO2 and zones of plant stress became evident after ruling out these other environmental possibilities for stress.
It can also be possible to use the spectral signature of vegetation to eliminate drought or prolonged exposure to high heat as possible stressors. Leaf water content strongly influences hyperspectral plant signatures outside the visible spectra (Carter 1991). Because this experiment took place during the end of a hot summer, there was a possibility that the vegetation was showing stress because they were simply drying out. To investigate this possibility, the Normalized Difference Water Index (NDWI) was calculated over the course of the injection for vegetation at various measurement sites across the east edge of the plant study area. NDWI values are calculated by the difference of the reflectance at 860 and 1,240 nm divided by their sum. NDWI has been found to correlate with water content of vegetation (Gao 1995). Figure 13 is a graph of NDWI for vegetation on the east edge of the plant study area located 0.5-, 1-, 2.5-, 7.5-m north, as well as 10-m south, of the injection well. Measurements from vegetation at 7.5 and 10 m from the injection well were outside the CO2 leaks and can be considered as background values. Figure 13 shows an overall decrease in water content for all vegetation across the field, suggesting that high summer heat has affected the water content of all the plants. However, the water content in vegetation within 2.5 m of the injection well decreased at a faster rate than background vegetation. This result indicates an additional stress, besides high heat, was acting on these plants because the temperature and precipitation were consistent between all areas of the field. In this experiment, the additional stress can be linked to the high CO2 flux within 2.5 m of the injection well because it is the only different factor known between the vegetation. However, in general, this connection cannot be made since the CO2 flux is usually unknown. Normalizing for water content can at least eliminate some stressors and reduce the chance of false positives for CO2 leaks. It is possible that other stressors could be eliminated similarly. Therefore, it is important to acquire hyperspectral data over an entire region and not just over pipelines and other potential leak sites of sequestration fields.
Plot of Normalized Difference Water Index (NDWI) values. Vegetation is located along east edge of plant study area at various distances from the injection well. Vegetation in the CO2 leak zone is plotted in red. Background values are in blue. NDWI = (ρ860 − ρ1240)/(ρ860 + ρ1230), ρ = reflectance at given wavelength in nm
Conclusions and recommendations
CO2 leaks through the soil could successfully be identified by hyperspectral signatures to determine the health of overlying vegetation. The plant spectra began to show signs of stress within 4 days of the CO2 injection. The lower limit of soil CO2 concentration needed to induce this stress can be estimated to be between 4 and 8% CO2 by volume. The spatial extent of plant stress, as determined by spectrometers in the field and from the air, match the locations of high measured CO2 flux from the injection.
There are also many additional benefits to this monitoring technique, such as a quick response time. Vegetation is very sensitive to stress, so a suspected leak could be identified within days. Also, the actual spectral measurements can be collected and processed rapidly by acquiring airborne hyperspectral imagery, which will have no impact on the sequestration field itself. More importantly, this monitoring technique can be utilized long term, even on 100-year timescales, as it would require little to no maintenance of the sequestration field. The only requirement is simply the presence of vegetation. With these benefits, analyzing hyperspectral plant signatures for stress is an efficient method to verify that sequestration fields are successful in retaining injected CO2.
Future work will focus on monitoring CO2 leaks with various instruments, particularly on airborne and/or satellite platforms. Work will also go toward determining the specific mechanisms that cause plant stress due to changes in soil ecosystems from a CO2 leak. In addition, the species dependence of the plant stress caused by elevated CO2 soil concentrations is another topic to be investigated.
References
Bateson L, Vellico M, Beaubien SE, Pearce JM, Annunziatellis A, Ciotoli G, Coren F, Lombardi S, Marsh S (2008) The application of remote-sensing techniques to monitor CO2-storage sites for surface leakage: method development and testing at Latera (Italy) where naturally produced CO2 is leaking to the atmosphere. Int J Greenhouse Gas Control 2:388–400. doi:10.1016/j.ijggc.2007.12.005
Blackburn GA (2007) Hyperspectral remote sensing of plant pigments. J Exp Bot 58:855–867. doi:10.1093/jxb/erl123
Carter GA (1991) Primary and secondary effects of water content on the spectral reflectance of leaves. Am J Bot 78:916–924. doi:10.2307/2445170
Carter GA (1993) Responses of leaf spectral reflectance to plant stress. Am J Bot 80:239–243. doi:10.2307/2445346
Carter GA, Knapp AK (2001) Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll concentration. Am J Bot 88:677–684. doi:10.2307/2657068
De Jong SM (1996) Surveying dead trees and CO2-induced stressed trees using AVIRIS in the Long Valley Caldera. In: Summaries of the sixth annual JPL airborne earth science workshop. JPL Publication 96, pp 67–74
Gao B (1995) Normalized difference water index for remote sensing of vegetation liquid water from space. Proc SPIE 2480:225. doi:10.1117/12.210877
Hausback BP, Strong M, Farrar C, Pieri D (1998) Monitoring of volcanogenic CO2-induced tree kills with AVIRIS image data at Mammoth Mountain, California. In: Summaries of the seventh annual JPL airborne earth science workshop. JPL Publication 98
Hill M (2004) Grazing agriculture: managed pasture, grassland, and rangeland. In: Ustin S (ed) Remote sensing for natural resources management and environmental monitoring: manual of remote sensing, vol 4, 3rd edn. Wiley, Hoboken, pp 449–530
Kruse FA, Keirein-Young KS, Boardman JW (1990) Mineral mapping at Cuprite, Nevada, with a 63-channel imaging spectrometer. Photogramm Eng Rem S 56:83–92
Lewicki JL, Hilley GE, Dobeck L, Spangler L (2009, this volume) Dynamics of CO2 fluxes and concentrations during a shallow subsurface CO2 release. Env Geol
Lichtenthaler HK (1998) The stress concept in plants: an introduction. Ann NY Acad Sci 851:187–198. doi:10.1111/j.1749-6632.1998.tb08993.x
Martini BA, Silver EA, Potts DC, Pickles WL (2000) Geological and geobotanical studies of Long Valley Caldera, CA, USA utilizing new 5 m hyperspectral imagery. Geoscience and remote sensing symposium, 2000 proceedings. IGARSS 2000. IEEE 2000 International 4:1376–1378. doi:10.1109/IGARSS.2000.857212
Moorthy I, Miller JR, Noland TL (2008) Estimating chlorophyll concentration in conifer needles with hyperspectral data: an assessment at the needle and canopy level. Remote Sens Environ 112:2824–2838. doi:10.1016/j.rse.2008.01.013
Noomen MF, Skidmore AK (2009) The effects of high soil CO2 concentrations on leaf reflectance of maize plants. Int J Remote Sens 30:481–497. doi:10.1080/01431160802339431
Noomen MF, Skidmore AK, Van Der Meer FD, Prins HH (2006) Continuum removed band depth analysis for detecting the effects of natural gas, methane and ethane on maize reflectance. Remote Sens Environ 105:262–270. doi:10.1016/j.rse.2006.07.009
Noomen MF, Smith KL, Colls JJ, Steven MD, Skidmore AK, Van Der Meer FD (2008) Hyperspectral indices for detecting changes in canopy reflectance as a result of underground natural gas leakage. Int J Remote Sens 29:5987–6008. doi:10.1080/01431160801961383
Pickles WL, Cover WA (2005) Hyperspectral geobotanical remote sensing for CO2 storage monitoring. In: Thomas DC, Benson S (eds) Carbon dioxide capture for storage in deep geologic formations—results from the CO2 capture project capture and separation of carbon dioxide from combustion sources, vol 2. Elsevier, San Diego, pp 1045–1070 (ISBN: 0-08-044570-5)
Smith KL, Steven MD, Colls JJ (2004) Use of hyperspectral derivatives ratios in the red-edge region to identify plant stress responses to gas leaks. Remote Sens Environ 92:207–217. doi:10.1016/j.rse.2004.06.002
Sohn Y, Rebello NS (2002) Supervised and unsupervised spectral angle classifiers. Photogramm Eng Rem S 68:1271–1280
Spangler LH, Dobeck L et al (2009) A controlled field pilot in Bozeman, Montana, USA, for testing near surface CO2 detection techniques and transport models. Env Geol
Zhang Y, Chen JM, Miller JR, Noland TL (2008) Leaf chlorophyll content retrieval from airborne hyperspectral remote sensing imagery. Remote Sens Environ 112:3234–3247. doi:10.1016/j.rse.2008.04.005
Acknowledgments
We thank Laura Dobeck and Kadie Gullickson of Montana State University for their help with the experiment layout. We also thank Lee Spangler of Montana State University and Rand Swanson of Resonon, Inc., in Bozeman, Montana. We acknowledge the Montana Board of Research and Commercialization Technology (Grant No. 08–44) and NASA (Grant No. NNX06AD11G) for funding of the airborne hyperspectral imagery. Martha Apple acknowledges support by the US Department of Energy EPSCoR program under (Grant No. DE-FG02-08ER46527) for plant response research.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Male, E.J., Pickles, W.L., Silver, E.A. et al. Using hyperspectral plant signatures for CO2 leak detection during the 2008 ZERT CO2 sequestration field experiment in Bozeman, Montana. Environ Earth Sci 60, 251–261 (2010). https://doi.org/10.1007/s12665-009-0372-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0372-2