Abstract
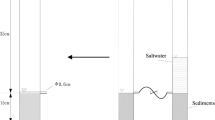
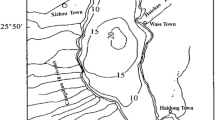
Mineralization of organic matter (OM) in sediment is crucial for biogeochemical cycle of nitrogen (N) and phosphorus (P) in lake ecosystem. Light fraction OM (LFOM) is a reactive pool in sediment and is considered as labile fraction contributing to N and P cycling. In our study, the effect of LFOM on the process and characteristics of N and P mineralization in sediments of Taihu Lake were investigated with 77-day waterlogged incubation plus intermittent leaching at 27°C. Sediments from Yuantouzhu (Y) and Gonghu (G) were used, which were removed the LF. Results indicated that the organic nitrogen mineralized ranged from 14.3 to 19.5% of total nitrogen (193.49–378.93 mg kg−1 sediment) and the organic phosphorus mineralized ranged from 5.7 to 7.9% of total phosphorus (19.86–60.65 mg kg−1 sediment). The heavily polluted sediment had a higher mineralization rate and net mineral-N and mineral-P than slightly polluted sediment. LF stimulated the initial amounts of inorganic N and P and also can be the potential source for mineralization. After the LFOM removal, the net mineral-N of Y and G decreased 116.47 mg kg−1 sediment and 48.03 mg kg−1sediment, respectively, and the net mineral-P decreased 2.67 mg kg−1sediment for Y and 4.82 mg kg−1sediment for G. Two models were used to fit the observed mineral-N data vs. incubation days using a non-linear regression procedure: one is the effective cumulated temperature model, a thermodynamic model which assumes that N mineralization is affected by temperature; the other is the single first-order exponential model, which is a dynamic model. Based on root mean square error values for the two models, the effective cumulated temperature model made a better prediction of N mineralization than the other model for all the four treatments. The single first-order exponential model underestimated N mineralization during the first 14 days and the last 21 days, and overestimated it in the other days during the 77-day incubation. This indicated that temperature was the primary factor influencing N mineralization and the amount of mineral-N were correlated significantly with the effective cumulated temperature (T ≥ 15°C) and incubation time when waterlogged incubation plus intermittent leaching was used.








Similar content being viewed by others
References
Acquaye DK (1963) Some significance of soil organic phosphorus mineralization in the phosphorus nutrition of cocoa in Ghana. Plant Soil 19:65–80
Bolalek J, Graca B (1996) Ammonia nitrogen at the water-sediment interface in Puck Bay (Baltic Sea). Estuar Coast Shelf Sci 43:767–779
Boone BD (1994) Light-fraction soil organic matter: origin and contribution to net nitrogen mineralization. Soil Biol Biochem 26:1459–1468
Bremer E, Janzen HH, Johnson AM (1994) Sensitivity of total, light fraction and mineralizable organic matter to management practices in a Lethbridge soil. Can J Soil Sci 74:131–138
Cai GX, Zhang SL, Zhu ZL (1979) Condition experiment of waterlogged incubation in determining paddy soil nitrogen mineralization. Soil 6:234–240
Christensen BT (1992) Physical fractionation of soil and organic matter in primary particle size and density separates. Adv Soil Sci 20:2–90
Cookson WR, Abaye DA, Marschner P et al (2005) The contribution of soil organic matter fractions to carbon and nitrogen mineralization and microbial community size and structure. Soil Biol Biochem 37:1726–1737
Curtin D, McCallum FM, Williams PH (2004) Phosphorus in light fraction organic matter separated from soils receiving long-term applications of superphosphate. Biol Fertil Soils 37:280–287
Dou Z, Toth JD, Jabro JD, Fox RH, Fritton DD (1996) Soil nitrogen mineralization during laboratory incubation: dynamics and model fitting. Soil Biol Biochem 28:625–632
Golchin A, Oades JM, Skjemstad JO (1994) Study of free and occurred particulate organic matter in soils by solid state 13C CP/MAS NMR spectroscopy and scanning electron microscopy. Aust J Soil Res 32:285–309
Greenland DJ, Ford GW (1964) Separation of partially humified organic materials from soils by ultrasonic dispersion. In: Transactions of the eighth International Congress of Soil Science, Bucharest, pp 137–148
Institute of Soil Science (IOSS), Chinese Academy of Sciences (1978) Soil physics and chemistry analyze. Shanghai Science and Technology Publisher, Shanghai (in Chinese)
Jana EC, Richard DB (2002) Soil nitrogen transformations and the role of light fraction organic matter in forest soils. Soil Biol Biochem 34:933–943
Janzen HH, Campbell CA, Brabdt SA (1992) Light-fraction organic matter in soils from long-term crop rotations. Soil Sci Soc Am J 56:1799–1806
Jin XC, Cui Z, Wang SR (2006) Nitrogen mineralization processes of sediments and soil under continuously waterlogged incubation conditions. Chin J Soil Sci 37:909–915
Kadono A, Funakawa S, Kosaki T (2008) Factors controlling mineralization of soil organic matter in the Eurasian steppe. Soil Biol Biochem 40:947–955
Keeney DR, Bremner JM (1996) Comparison and evaluation of laboratory methods of obtaining an index of soil nitrogen availability. Agron J 58:498–503
Li SX, Ai SY, He H (1999) Soil’s nitrogen mineralization processes under continuously waterlogged incubation conditions. Acta Univ Agric Boreali-occidentalis Sin 27:1–5
Li HL, Han Y, Cai ZC (2003) Nitrogen mineralization in paddy soils of the Taihui Region of China under anaerobic conditions: dynamics and model fitting. Geoderma 115:161–175
Liu HL, Jin XC, Jing YF (1999) Environmental dredging technology of lake sediment. Chin Eng Sci 1:81–84 (in Chinese)
Molina JAE (1980) Potentially mineralization nitrogen in soil: the simple exponential model does not apply for the first 12 weeks of incubation. Soil Sci Soc Am J 44:996–1000
Monaghan R, Barraclough D (1995) Contributions to gross N mineralization from N-15-labelled soil macroorganic matter fractions during laboratory incubation. Soil Biol Biochem 27:1623–1628
Monaghan R, Barraclough D (1997) Contributions to N mineralization from soil macroorganic matter fractions incorporated into two field soils. Soil Biol Biochem 29:1215–1223
Mu XM, Fan XL (1999) A review on ecological models of soil N mineralization. Chin J Appl Ecol 10:114–118
Ni JZ, Xu JM, Xie ZM (2000) Light fraction organic matter in soil. Tech Equip Environ Pollut Control 1:58–63
Nierop KGJ, Preston CM, Verstraten JM (2006) Linking the B ring hydroxylation pattern of condensed tannins to C, N and P mineralization. A case study using four tannins. Soil Biol Biochem 38:2794–2802
Osono T, Takeda H (2004) Accumulation and release of nitrogen and phosphorus in relation to lignin decomposition in leaf litter of 14 tree species. Ecol Res 19:593–602
Penn MR, Auer MT, Van Oiman EL et al (1995) Phosphorus diagenesis in lake sediment: investigation using fractionation techniques. Mar Freshw Res 46:89–99
Ruban V, Lopez-Sanchez JF, Pardo P et al (2001) Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—a synthesis of recent works. Fresenius J Anal Chem 370:224–228
Ryan JL, Sims JL, Feaslee DE (1972) Laboratory methods for estimating plant available nitrogen in soil. Agron J 63:48–51
Saggar S, Parfitt RL, Salt G et al (1998) Carbon and phosphorus transformations during decomposition of pine forest floor with different phosphorus status. Biol Fertil Soils 27:198–204
Sollins P, Spycher G, Glassman CA (1984) Net nitrogen mineralization from light and heavy-fraction forest soil organic matter. Soil Biol Biochem 16:31–37
Stanford G, Smith SJ (1972) Nitrogen mineralization potentials of soils. Soil Sci Soc Am J 36:465–470
State Environmental Protection Administration (SEPA) (2002) Monitoring and analysis methods of water and wastewater, 4th edn. China Environmental Sciences Press, Beijing
Sundby B, Gobeil C, Silberberg N (1992) The phosphorus cycle in coastal marine sediments. Limnol Oceanogr 37:1129–1145
Tao QN, Wu LH, Fang P (1993) Study on mineralization rate of nitrogen in Paddy soils. Acta Pedol Sin 30:237–244
Wang YH, Jiang SZ, Gu YM (1983) A study on predicting nitrogen supplying capacity of gleyed paddy soil in the suburbs of Shanghai. Acta Pedol Sin 20:263–271
Wang SR, Jin XC, Pang Y et al (2005) Phosphorus fractions and phosphate sorption characteristics in relation to the sediment compositions of shallow lakes in the middle and lower reaches of Yangtze River region, China. J Colloid Interface Sci 289:339–346
Wang J, Wang SR, Jin XC et al (2007a) Ammonium release characteristics of the sediments from the shallow lakes in the middle and lower reaches of Yangtze River region, China. Environ Geol. doi:10.1007/s00254-007-0962-9
Wang SR, Jin XC, Zhao HC et al (2007b) Effect of organic matter on the sorption of dissolved organic and inorganic phosphorus in lake sediments. Colloids Surf A Physicochem Eng Asp 297:154–162
Warning SA, Bremner JM (1964) Ammonium production in soil under waterlogged conditions as an index of nitrogen availability. Nature 201:951–952
Whalen JK, Bottomley PJ, Myrold DD (2000) Carbon and nitrogen mineralization from light- and heavy-fraction additions to soil. Soil Biol Biochem 32:1345–1352
Wu TY, Schoenau JJ, Li FM et al (2005) Concepts and relative analytical techniques of soil organic matter. Chin J Appl Ecol 15:717–722
Zhu ZL, Cai GX (1984) Nitrogen mineralization of paddy soils in Tai-Lake region and the prediction of soil nitrogen supply. Acta Pedol Sin 21:29–36
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No. 40703017, 40873079) and Basic Scientific Research of Chinese Research Academy of Environment Sciences (No. 2007KYYW27).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, C., Wang, S., Jin, X. et al. Nitrogen and phosphorus mineralization in sediments of Taihu Lake after the removal of light fraction organic matter. Environ Earth Sci 59, 1437–1446 (2010). https://doi.org/10.1007/s12665-009-0130-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0130-5