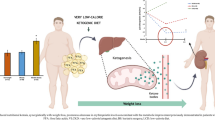
Abstract
Background
Conventional therapy can result in remission in mild-moderate pediatric Crohn’s disease (CD). However, some patients experience loss of response to biological drugs despite increased dosage.
Methods
We planned to determine that CD exclusion diet plus partial enteral nutrition offers additional benefits in asymptomatic children with CD having elevated fecal calprotectin. A randomized, open-label, pilot, controlled interventional study was conducted in children with CD while on medical treatment and elevated fecal calprotectin on routine testing. Patients continued their medications and were randomized into a group that received CD exclusion diet plus partial enteral nutrition for 12 weeks and one that continued a regular diet.
Results
Twenty-one patients participated: 11 received CD exclusion diet plus partial enteral nutrition and 10, regular diet. Median fecal calprotectin in the CD exclusion diet plus partial enteral nutrition decreased in 9/11 to 50% of baseline, remaining practically unchanged in the regular diet, except for two patients (p = 0.005). Body mass index z-score increased in the CD exclusion diet plus partial enteral nutrition. Only 1/11 patients in the CD exclusion diet plus partial enteral nutrition group, while 4/10 in the regular diet, experienced clinical relapse (p = 0.149). Only one patient in the CD exclusion diet plus partial enteral nutrition, while eight in the regular diet, were considered to need their biologic treatment intensified (p = 0.005); 2/11 in the CD exclusion diet plus partial enteral nutrition had the dose or frequency of the biologic reduced vs. none (0/10) in the regular diet group. The short Pediatric Crohn’s Disease Activity Index and anthropometry showed no significant changes in either group.
Conclusions
Diet therapy could be a useful addition to medications in children with CD in apparent remission, but elevated fecal calprotectin.
Trial registration
Clinical trial number: NCT05034458.
Graphical abstract




Similar content being viewed by others
References
Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863–73. https://doi.org/10.1053/j.gastro.2006.12.003.
Sigall Boneh R, Sarbagili Shabat C, Yanai H, et al. Dietary therapy with the Crohn’s disease exclusion diet is a successful strategy for induction of remission in children and adults failing biological therapy. J Crohns Colitis. 2017;11:1205–12. https://doi.org/10.1093/ecco-jcc/jjx071.
Scarallo L, Banci E, Pierattini V, Lionetti P. Crohn’s disease exclusion diet in children with Crohn’s disease: a case series. Curr Med Res Opin. 2021;37:1115–20. https://doi.org/10.1080/03007995.2021.1920901.
Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157:440–50.e8. https://doi.org/10.1053/j.gastro.2019.04.021.
Laharie D, D'Haens G, Nachury M, et al. Steroid‐free deep remission at one year does not prevent Crohn’s disease progression: long‐term data from the TAILORIX trial. Clin Gastroenterol Hepatol. 2022; 20:2074–82; https://doi.org/10.1016/j.cgh.2021.11.030
Elhag DA, Kumar M, Saadaoui M, et al. Inflammatory bowel disease treatments and predictive biomarkers of therapeutic response. Int J Mol Sci. 2022;23:6966. https://doi.org/10.3390/ijms23136966.
García-Sánchez V, Iglesias-Flores E, González R, et al. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis. 2010;4:144–52. https://doi.org/10.1016/j.crohns.2009.09.008.
Koninckx CR, Donat E, Benninga MA, et al. The use of fecal calprotectin testing in paediatric disorders: a position paper of the European Society for Paediatric Gastroenterology and Nutrition Gastroenterology Committee. J Pediatr Gastroenterol Nutr. 2021;72:617–40. https://doi.org/10.1097/MPG.0000000000003046.
Liu F, Lee SA, Riordan SM, et al. Global studies of using fecal biomarkers in predicting relapse in inflammatory bowel disease. Front Med. 2020;17:580803. https://doi.org/10.3389/fmed.2020.580803.
Kneißl S, Stallhofer J, Schlattmann P, Stallmach A. Disease recurrence in patients with Crohn’s disease after biologic therapy or surgery: a meta-analysis. Int J Colorectal Dis. 2022;37:2185–95. https://doi.org/10.1007/s00384-022-04254-z.
Levine A, Koletzko S, Turner D, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. https://doi.org/10.1097/MPG.0000000000000239.
Sigall-Boneh R, Pfeffer-Gik T, Segal I, et al. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm Bowel Dis. 2014;20:1353–60. https://doi.org/10.1097/MIB.0000000000000110.
Turner D, Griffiths AM, Walters TD, et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis. 2012;18:55–62. https://doi.org/10.1002/ibd.21649.
de Onis, Mercedes. Length for age, weight for age and body mass index for age: methods and development in WHO child growth standards. World Health Organisation. 2006. https://www.who.int/publications/i/item/924154693X. Accessed May 6, 2023.
Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification of inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–21. https://doi.org/10.1002/ibd.21493.
Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–83. https://doi.org/10.1053/j.gastro.2020.12.031.
Chew TS, Mansfield JS. Can faecal calprotectin predict relapse in inflammatory bowel disease: a mini review. Frontline Gastroenterol. 2018;9:23–8. https://doi.org/10.1136/flgastro-2016-100686.
Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013;7:e641–51. https://doi.org/10.1016/j.crohns.2013.05.005.
Deberry R, Rahman A, Dubois P, et al. PTU-080 Calprotectin predicts relapse of ibd even in the presence of a ‘normal’ colonoscopy. Gut. 2015;64:A95–6. https://doi.org/10.1136/gutjnl-2015-309861.195.
van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2020;7:jjaa161. https://doi.org/10.1093/ecco-jcc/jjaa161.
Matuszczyk M, Meglicka M, Wiernicka A, et al. Effect of the Crohn’s disease exclusion diet (CDED) on the fecal calprotectin level in children with active Crohn’s disease. J Clin Med. 2022;11:4146. https://doi.org/10.3390/jcm11144146.
Scarallo L, Lionetti P. Dietary management in pediatric patients with Crohn’s disease. Nutrients. 2021;13:1611. https://doi.org/10.3390/nu13051611.
Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammatory bowel disease. J Hum Nutr Diet. 2011;24:313–26. https://doi.org/10.1111/j.1365-277X.2011.01171.x.
Nguyen DL, Palmer LB, Nguyen ET, et al. Specialized enteral nutrition therapy in Crohn’s disease patients on maintenance infliximab therapy: a meta-analysis. Therap Adv Gastroenterol. 2015;8:168–757. https://doi.org/10.1177/1756283X15578607.
Ashton JJ, Gavin J, Beattie RM. Exclusive enteral nutrition in Crohn’s disease: evidence and practicalities. Clin Nutr. 2019;38:80–9. https://doi.org/10.1016/j.clnu.2018.01.020.
Shah R, Kellermayer R. Microbiome associations of therapeutic enteral nutrition. Nutrients. 2014; 6:5298–311; https://doi.org/10.3390/nu6115298
Gu P, Feagins LA. Dining with inflammatory bowel disease: a review of the literature on diet in the pathogenesis and management of IBD. Inflamm Bowel Dis. 2020;26:181–91. https://doi.org/10.1093/ibd/izz268.
Logan M, Clark CM, Ijaz UZ, et al. The reduction of faecal calprotectin during exclusive enteral nutrition is lost rapidly after food re-introduction. Aliment Pharmacol Ther. 2019;50:664–74. https://doi.org/10.1111/apt.15425.
Sahu P, Kedia S, Ahuja V, Tandon RK. Diet and nutrition in the management of inflammatory bowel disease. Indian J Gastroenterol. 2021;40:253–64. https://doi.org/10.1007/s12664-021-01163-x.).
Acknowledgements
The authors wish to thank Anabella Famiglietti, R.D., from the Hospital Italiano of Buenos Aires and Rocio Viollaz, R.D., from the Hospital Sor Ludovica de la Plata, La Plata, Provincia de Buenos Aires, Argentina, for dietary counseling to patients in the study; Vanina Pagotto, M.D., from the Hospital Italiano of Buenos Aires, for statistical analyses, Nestle Health Science Argentina for the donation of the special formula used, Leigh Emerson, Robert Shulman, M.D. and Richard Kellermayer, M.D., for editorial assistance and critical review of the manuscript.
Funding
This research was funded in part through an International Cooperation Grant in Child Nutrition of the Spanish Society of Gastroenterology, Hepatology and Pediatric Nutrition 2021 and through the Young Investigator Award of the Latin American Society of Gastroenterology, Hepatology and Pediatric Nutrition 2022.
Author information
Authors and Affiliations
Contributions
Maria Soledad Arcucci: study design, data collection, data interpretation, writer of the first manuscript draft, editing and approval of final manuscript. Lorena Menendez: study design, data collection, data interpretation, manuscript editing and approval of final manuscript. Marina Orsi: study design, data interpretation, manuscript editing and approval of final manuscript. Julieta Gallo: study design, data collection, data interpretation, manuscript editing and approval of final manuscript. Luciana Guzman: study design, data collection, data interpretation, manuscript editing and approval of final manuscript. Veronica Busoni: study design, data interpretation, manuscript editing and approval of final manuscript. Carlos Lifschitz: study design, data interpretation, manuscript editing and approval of final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MSA, LM, MO, JG, LG, VB and CL declare no competing interests.
Ethics statement
The study was performed conforming to the Helsinki Declaration of 1975, as revised in 2000 and 2008 concerning human and animal rights, and the authors followed the policy concerning informed consent as shown on Springer.com.
Disclaimer
The authors are solely responsible for the data and the content of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/ findings and content of this article.
Additional information
Trial identification number
PRIISA.BA https://www.buenosaires.gob.ar/salud/docencia-investigacion-y-desarrollo-profesional/priisaba/registro-publico-de-investigaciones number 4384.
Clinical trials
https://clinicaltrials.gov/ct2/show/NCT05034458?term=arcucci&cond=Crohn+Disease&cntry=AR&draw=2&rank=1 number 5922
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arcucci, M.S., Menendez, L., Orsi, M. et al. Role of adjuvant Crohn’s disease exclusion diet plus enteral nutrition in asymptomatic pediatric Crohn’s disease having biochemical activity: A randomized, pilot study. Indian J Gastroenterol 43, 199–207 (2024). https://doi.org/10.1007/s12664-023-01416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-023-01416-x