Abstract
Background and Aims
Functional constipation is a common childhood problem, with a prevalence of approximately 3% worldwide. The aim of the study was to compare the efficacy of polyethylene glycol (PEG) 3350 and lactulose in the treatment of pediatric functional constipation.
Methods
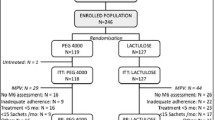
A total of 100 subjects with functional constipation were enrolled and centrally randomized to receive PEG 3350 (0.7–1.5 mg/kg/day) or lactulose (0.7–2.0 g/kg/day).
Results
There was a significant increase in median (min, max) stool frequency within 1 week in the PEG 3350 group as compared to the lactulose group (1 [0, 3] to 8 [3, 39] vs. 1 [0, 3] to 7 [1, 17]) (p-value < 0.01). The trend was maintained at week 2, week 3 (p-value < 0.01), and week 4 (p-value = 0.05) with the PEG 3350 group reporting higher weekly median stool frequency than the lactulose group. The PEG group reported significant reduction in painful bowel movements from 68.8% subjects at baseline to 43.8% at the end of first week, whereas the lactulose group reported an increase from 48.9% to 73.3% (p-value = 0.05). Other parameters of constipation, i.e. straining, large diameter stool, and large fecal mass as reported subjectively by parents, significantly decreased from baseline to the end of the study in the PEG 3350 arm compared to those in the lactulose arm. At the end of week 4, there was a statistically significant reduction in all the ROME IV–defined criteria between the two groups.
Conclusion
This study proved that the PEG 3350 treatment group had early symptom relief and significant improvement compared to the lactulose group in pediatric functional constipation.
Trial registration
Clinical Trials Registry India (CTRI/2018/01/011061)

Similar content being viewed by others
References
Rowan-Legg A, Canadian Paediatric Society, Community Paediatrics Committee. Managing functional constipation in children. Paediatr Child Health. 2011;16:661–70.
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Evaluation and treatment of constipation in children: summary of updated recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2006;43:405–7.
van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006;101:2401–9.
Borowitz SM, Cox DJ, Kovatchev B, et al. Treatment of childhood constipation by primary care physicians: efficacy and Predictors of outcome. Pediatrics. 2005;115:873–7.
Benninga MA, Voskuijl WP, Taminiau JA. Childhood constipation: is there new light in the tunnel? J Pediatr Gastroenterol Nutr. 2004;39:448–64.
Rubin G, Dale A. Chronic constipation in children. BMJ. 2006;333:1051–5.
Poddar U. Approach to constipation in children. Indian Pediatr. 2016;53:319–27.
Hyams JS, Di Lorenzo C, Saps M, et al. Childhood functional gastrointestinal disorders: child/ adolescent. Gastroenterology. 2016;150:1456–68.
Lee YJ, Park KS. Understanding the changes in diagnostic criteria for functional constipation in pediatric patients: from Rome III to Rome IV. J Neurogastroenterol Motil. 2019;25:3–5.
Bekkali NLH, Hoekman DR, Liem O, et al. Polyethylene glycol 3350 with electrolytes versus polyethylene glycol 4000 for constipation: a randomized, controlled trial. J Pediatr Gastroenterol Nutr. 2018;66:10–5.
Jarzebicka D, Sieczkowska-Golub J, Kierkus J, et al. PEG 3350 versus lactulose for treatment of functional constipation in children: randomized study. J Pediatr Gastroenterol Nutr. 2019;68:318–24.
Voskuijl W, de Lorijn F, Verwijs W, et al. PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial. Gut. 2004;53:1590–4.
Pashankar DS, Bishop WP. Efficacy and optimal dose of daily polyethylene glycol 3350 for treatment of constipation and encopresis in children. J Pediatr. 2001;139:428–32.
Loening-Baucke V. Polyethylene glycol without electrolytes for children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 2002;34:372–7.
Youssef NN, Peters JM, Henderson W, et al. Dose response of PEG 3350 for the treatment of childhood fecal impaction. J Pediatr. 2002;141:410–4.
Lee-Robichaud H, Thomas K, Morgan J, Nelson RL. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev. 2010;7:CD007570.
Koppen IJ, Lammers LA, Benninga MA, Tabbers MM. Management of functional constipation in children: therapy in practice. Paediatr Drugs. 2015;17:349–60.
Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58:258–74.
Yachha SK, Srivastava A, Mohan N, et al. Management of childhood functional constipation; consensus practice guidelines of Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition. Indian Pediatr. 2018;55:885–92.
Treepongkaruna S, Simakachorn N, Pienvichit P, et al. A randomised, double-blind study of polyethylene glycol 4000 and Lactulose in the treatment of constipation in children. BMC Pediatr. 2014;19:153.
Gordon M, MacDonald JK, Parker CE, Akobeng AK, Thomas AG. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev. 2016;2016(8):CD009118.
Acknowledgements
The authors thank Fourrts India for providing the investigational products and logistic support for the conduct of the study. The authors would also like to thank Medclin Research for support in statistical analysis of data and in preparation of the initial draft of the manuscript.
Source of support: Investigational products were provided by the sponsor of the study: Fourrts India Pvt. Ltd.
Author information
Authors and Affiliations
Contributions
Nirmala Dheivamani: study concept and protocol design, collecting data; critical revision of the manuscript for intellectual content; study supervision. Winston Thomas: study concept and protocol design, collecting data, critical revision of the manuscript for intellectual content; study supervision. Rohit Bannerjii: collecting data, study supervision. Mallar Mukherjee: collecting data, study supervision. Monjori Mitra: study concept and protocol design; collecting data; analysis of data; preparing the initial draft of the manuscript; critical revision of the manuscript for intellectual content; study supervision.
Corresponding author
Ethics declarations
Conflict of interest
ND, WT, RB, and MK declare that they have no conflict of interest. Dr. Monjori Mitra, Professor of Pediatrics, Institute of Child Health, Kolkata, is also the Research Director of Medclin Research, Kolkata.
Ethics approval and consent to participate
The authors declare that the study was performed in a manner conforming to the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning human and animal rights. The study protocol was approved by the Institutional Ethics Committee, Institute of Child Health, Kolkata (18 Sep 2018) and Institutional Ethics Committee, Madras Medical College, Chennai (7 Nov 2018) and the trial was also registered in Clinical Trials Registry of India (CTRI/2018/01/011061). Consent was obtained from the subjects’ parents after full explanation of the purpose, nature, and risks of all procedures used.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way, the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers are responsible for the results/findings and content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dheivamani, N., Thomas, W., Bannerjii, R. et al. Efficacy of polyethylene glycol 3350 as compared to lactulose in treatment of ROME IV criteria–defined pediatric functional constipation: A randomized controlled trial . Indian J Gastroenterol 40, 227–233 (2021). https://doi.org/10.1007/s12664-021-01148-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-021-01148-w